2.5 Supplement Labels
As you learned last week, according to the Dietary Supplement Health and Education Act of 1994, the supplement industry is regulated by the FDA but supplements are regulated under food law. Any product that meets the legal definition of a dietary supplement is required by law to include a supplement facts panel. The exception to this is protein supplements, which have the option of using either a nutrition facts label or supplement facts label. The supplement facts label is similar to a nutrition facts label that you see on foods but there are a few differences. Figure 2.9 shows an example of a supplement facts panel.
Figure 2.9 Supplement Facts
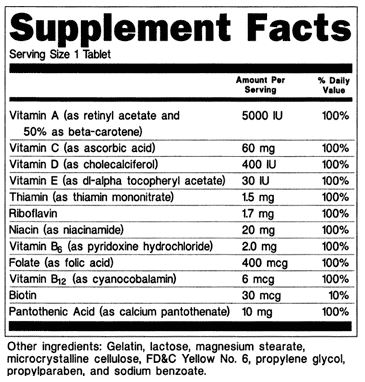
Here are some of the key requirements for supplement facts labels (1):
- The names and quantities of dietary ingredients present in the product, the “Serving Size” and the “Servings Per Container” are required to be listed on the label. If the servings per container are the same as the contents of the package, then servings per container may be omitted. For example, if there are 100 tablets in a package and the serving size is 1 tablet, then servings per container does not need to be included.
- Total calories, calories from fat, total fat, saturated fat, cholesterol, sodium, total carbohydrate, dietary fiber, sugars, protein, vitamin A, vitamin C, calcium, and iron must be listed when they are present in measurable amounts. Manufacturers are not required to list these nutrients if they can be listed as “zero” according to the nutrient content claims discussed earlier in this reading.
- Percent daily value (DV) must be listed for all nutrients that have an established DV. Supplements can provide more than 100% of the DV for nutrients. However, the label is not required to indicate if a supplement provides more than the UL for the nutrient. If you take vitamin or mineral supplements, it is recommended that you check the UL for each nutrient to be sure that you are not consuming any nutrients in quantities that may cause toxicity. You can find the UL for each micronutrient in the DRI tables linked earlier in this reading.
- Manufacturers recommended dose and instructions for use must be included. Savvy consumers should know that there is no requirement that the manufacturer’s recommendation be for a dose that has been shown to be effective.
- All ingredients in a proprietary blend must be listed, however, the exact amount of each ingredient does not need to be disclosed. This is to prevent competitors from knowing the exact formula. But also prevents consumers from knowing exactly what is in their supplements.
- Structure/function claims are allowed. However, these claims are not evaluated by the FDA and must include a disclaimer. Manufacturers often use misleading statements to make their products appear better and hook consumers with these too good to be true promises.
Media Attributions
- Supplement Facts © FDA is licensed under a Public Domain license
