6.2 Phospholipids and Sterols
Phospholipids
Phospholipids make up only about 2 percent of dietary lipids. They are partially water-soluble and partially fat-soluble and are found in both plants and animals. Phospholipids are crucial for building the protective barrier, or membrane, around your body’s cells. In fact, phospholipids are synthesized in the body to form cell and organelle membranes. In blood and body fluids, phospholipids form structures in which fat is enclosed and transported throughout the bloodstream. Figure 6.9 shows the chemical structure of a phospholipid.
Figure 6.9 Chemical Structure of a Phospholipid
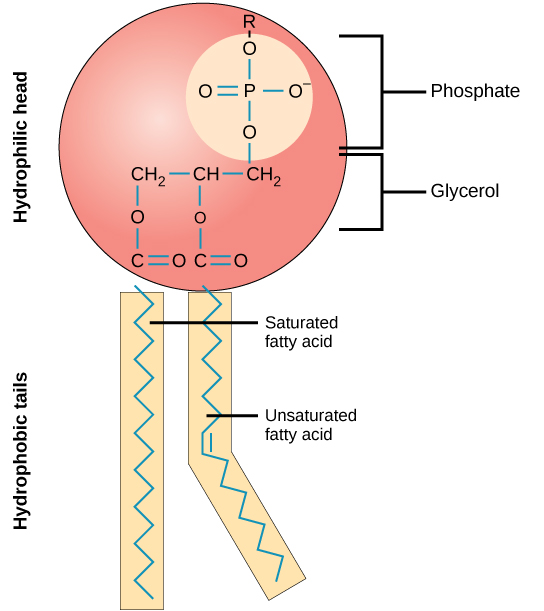
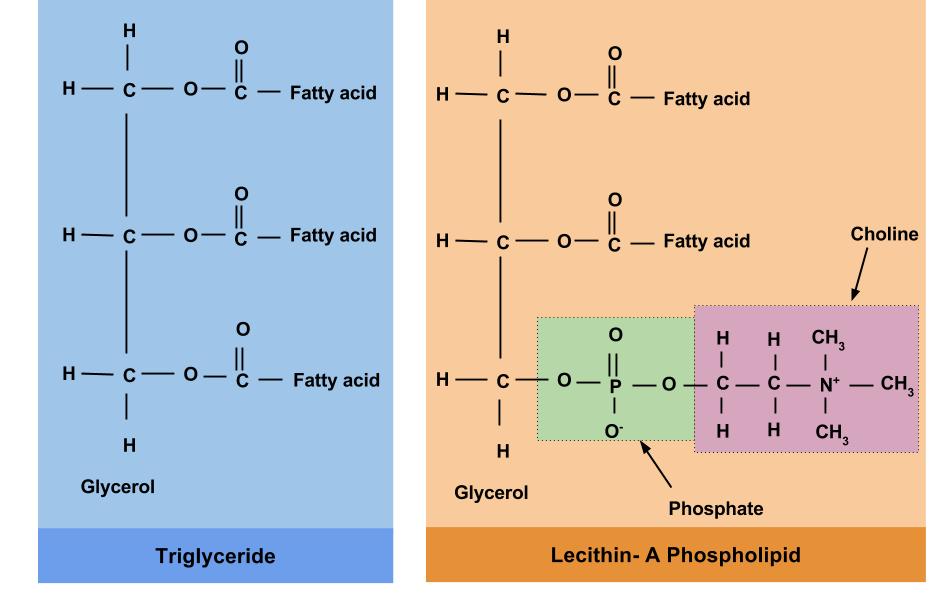
Like triglycerides, phospholipids have a glycerol backbone. But unlike triglycerides, phospholipids are diglycerides (two fatty-acid molecules attached to the glycerol backbone) with a phosphate group in the place of the third fatty acid on a triglyceride. This unique structure makes phospholipids water soluble. Phospholipids are what we call amphipathic, or a compound that can bind with both lipid and water. The fatty-acid tails are lipophilic (likes lipids) and the phosphate group is hydrophilic (likes water). Figure 6.10 compares the chemical structure of triglycerides to phospholipids.
Figure 6.10 Triglycerides vs Phospholipids
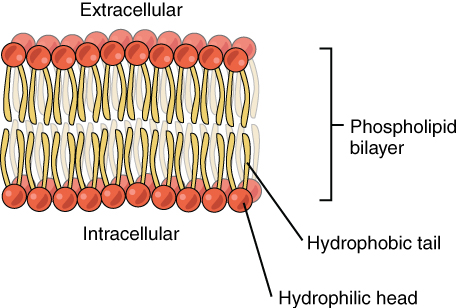
In the body phospholipids bind together to form cell membranes. The amphipathic nature of phospholipids governs their function as components of cell membranes. The phospholipids form a double layer in cell membranes, thus effectively protecting the inside of the cell from the outside environment while at the same time allowing for transport of fat and water through the membrane.
Figure 6.11 The Phospholipid Bilayer
Phospholipids are ideal emulsifiers that can keep oil and water mixed. Emulsions are mixtures of two liquids that do not mix. Without emulsifiers, the fat and water content would be somewhat separate within food. Lecithin (phosphatidylcholine), found in egg yolk, honey, and mustard, is a popular food emulsifier. Mayonnaise demonstrates lecithin’s ability to blend vinegar and oil to create the stable, spreadable condiment that so many enjoy. Food emulsifiers play an important role in making the appearance of food appetizing. Adding emulsifiers to sauces and creams not only enhances their appearance but also increases their freshness.
Lecithin’s crucial role within the body is clear, because it is present in every cell throughout the body; 28 percent of brain matter is composed of lecithin and 66 percent of the fat in the liver is lecithin. Many people attribute health-promoting properties to lecithin, such as its ability to lower blood cholesterol and aid with weight loss. There are several lecithin supplements on the market broadcasting these claims. However, as the body can make most phospholipids, it is not necessary to consume them in a pill. The body makes all of the lecithin that it needs.
Sterols
Sterols are the least common type of lipid. Cholesterol is perhaps the best well-known sterol. Though cholesterol has a notorious reputation, the body gets only a small amount of its cholesterol through food—most cholesterol is produced in the liver. Cholesterol is an important component of the cell membrane and is required for the synthesis of sex hormones, vitamin D, and bile salts. Figure 6.12 shows the chemical composition of cholesterol. Notice that this looks much different than the other lipids you’ve learned about so far.
Figure 6.12 Chemical Structure of Cholesterol
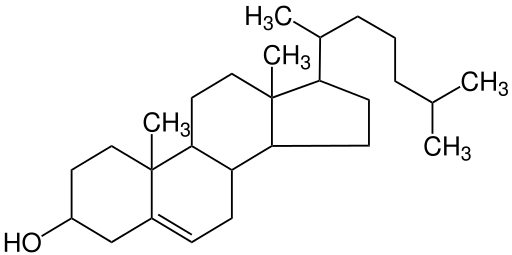
Sterols have a very different structure from triglycerides and phospholipids. Sterols do not contain fatty acids; instead they are composed of carbon, hydrogen, and oxygen arranged in multi ring structures. They are complex molecules that contain interlinking rings of carbon atoms, with side chains of carbon, hydrogen, and oxygen attached.
ecause cholesterol is produced by the liver, it is considered non-essential meaning it does not need to be obtained from the diet. Cholesterol is found in animal products because other animals (that also have livers) produce cholesterol too. Cholesterol is often linked to heart disease; however, this association is likely because cholesterol is often found in the same foods that have high amounts of saturated fat. We used to assume that eating foods high in cholesterol would lead to higher levels of artery clogging cholesterol in the blood. However, in healthy individuals, the liver produces less cholesterol when high amounts of dietary cholesterol are eaten so blood cholesterol is not proportionate to dietary cholesterol. Conversely, high amounts of saturated fat interfere with the normal processing of cholesterol in the body and as a result, less cholesterol is removed from the bloodstream by the liver. Removing the fat from foods is one way to reduce dietary cholesterol, but it is not possible to completely eliminate cholesterol from animal products that we eat. Foods that have the highest amounts of cholesterol are fatty meats, organ meats, eggs, and shellfish such as shrimp and lobster. All of the cholesterol in an egg is contained in the yolk, so eating egg whites only does not contribute to your cholesterol intake. However, egg yolks are excellent sources of many micronutrients so it is no longer recommended for most people to eliminate egg yolks from their diets.
Media Attributions
- Chemical Structure of a Phospholipid © Lumen Learning Intro to Chemistry is licensed under a CC BY-SA (Attribution ShareAlike) license
- Triglycerides vs Phospholipids © Allison Calabrese is licensed under a CC BY (Attribution) license
- Phospholipid Bilayer © Lumen Learning Intro to Chemistry is licensed under a CC BY-SA (Attribution ShareAlike) license
- Cholesterol Chemical Structure © Wesalius is licensed under a Public Domain license
