7.1 Structure and Function
Protein is another major macronutrient. They are similar to carbohydrates in that they are made up of small repeating units. But instead of sugars, proteins are made up of amino acids. Amino acids are composed of the elements carbon, hydrogen, oxygen, and nitrogen. Both carbohydrates and lipids are created from carbon, hydrogen, and oxygen. This makes proteins unique because carbohydrates can be converted to fats and vice versa but no other macronutrient can be converted to proteins because the other macronutrients lack nitrogen.
When people think of protein, they often think of eating meat and building muscle. This is technically true, but there are many plant based sources of protein and many functions of protein in addition to building and maintaining muscle mass. Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. They are the structural component of all tissues in the human body, this includes the proteins actin and myosin that cause your muscles to contract, keratin found in hair/skin/nails, and collagen found in your skin and connective tissue. Proteins also function as hormones, enzymes, antibodies, transporters in the blood (lipoproteins, hemoglobin, etc.), and neurotransmitters. In addition, proteins are involved in clotting reactions and fluid/pH balance in the blood. These are all considered primary functions of protein.
If we think back to the function of the other two macronutrients, we see that protein is very different. Carbohydrate is your body’s preferred source of energy. Fat is your body’s primary source of stored energy. As was discussed in chapter 1, 1 gram of protein provides 4 kcals, so the final function of protein is to provide energy. However, we say that providing energy is a secondary function of protein. Before protein is used to provide energy, it must first be deaminated. Deamination refers to the removal of the animo, or nitrogen containing, group which must take place before protein can be used for energy, converted to glucose via gluconeogenesis, or stored as fat. Your body prefers to use carbohydrate and fat for energy which frees up your amino acids to be used for their primary functions. Someone following a chronic low-calorie, low-carbohydrate diet may find it difficult to maintain lean muscle mass. This is because the protein that would have gone to building muscle is broken down to provide energy for the body. Even though protein is a very important nutrient, it functions best when there is adequate intake of all three macronutrients.
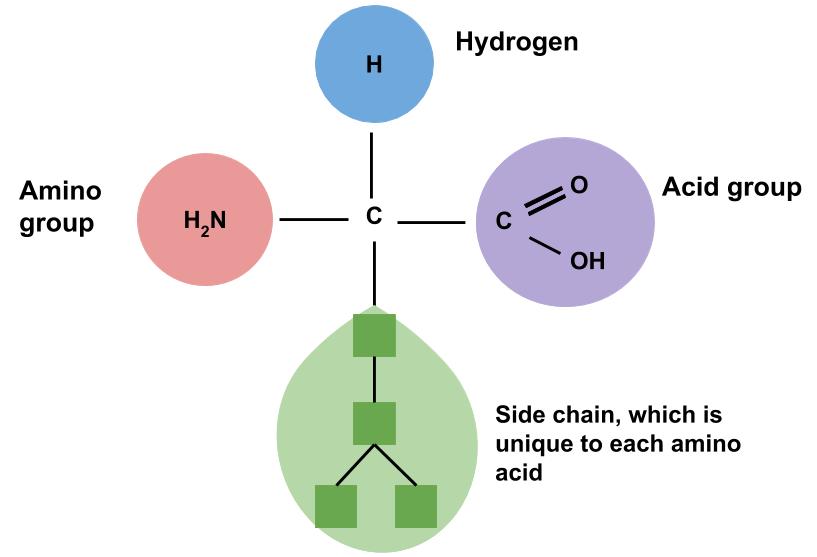
The human body contains hundreds of thousands of unique proteins. The functions of proteins are very diverse because there are 20 distinct amino acids that form long chains. Each cell in a living system may contain thousands of different proteins, each with a unique function. Their structures, like their functions, vary greatly. They are all, however, amino acids arranged in a linear sequence. As mentioned previously, amino acids are the building blocks of proteins. This name, amino acid, signifies that each contains an amine and an acid connected by a carbon backbone. Figure 7.2 shows the basic chemical structure of an amino acid. The only structural difference between each of the 20 amino acids is the side chain.
Figure 7.2 Chemical Structure of an Amino Acid
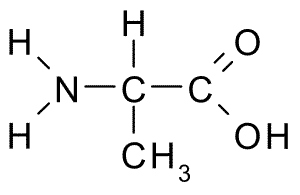
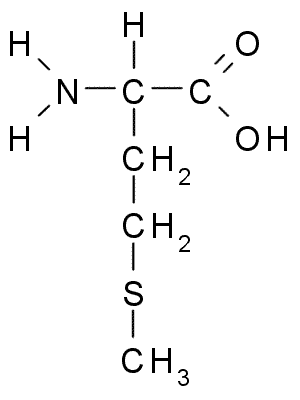
An example of the differences in the side group can be seen in Figure 7.3. Both alanine and methionine have amino groups (NH2), acid groups (COOH), and a hydrogen (H). However, the side chain on alanine (CH3) looks very different from methionine’s side chain (C3H7S).
Figure 7.3 Chemical Structures of Alanine (top) and Methionine (bottom)
Amino acids are classified as essential amino acids or non-essential amino acids. Non-essential amino acids can be produced by the body through a process called transamination. Transamination refers to the transfer of a nitrogen containing amino acid to a carbon skeleton (acid group and side chain). As long as there is adequate total nitrogen (protein) intake in the diet, a person will not be deficient in the non-essential amino acids. Non-essential amino acids are still necessary for proper body function, they just can be created in the body so are not essential to obtain from the diet. Essential amino acids cannot be made in the body. They must be obtained from the diet. A few amino acids are considered conditionally essential, which means under certain conditions such as extreme stress, illness, or premature infants the body is no longer able to produce them. Table 7.1 lists the essential and non-essential amino acids. Sometimes essential and non-essential amino acids are referred to as indispensable and dispensable amino acids.
Table 7.1 Essential and Non-Essential Amino Acids
| Essential Amino Acids | Non-Essential Amino Acids |
| Histidine | Alanine |
| Isoleucine | Arginine* |
| Leucine | Asparagine |
| Lysine | Aspartic acid |
| Methionine | Cysteine* |
| Phenylalanine | Glutamic acid |
| Threonine | Glutamine* |
| Tryptophan | Glycine* |
| Valine | Proline* |
| Serine | |
| Tyrosine* |
*conditionally essential
Media Attributions
- Chemical Structure of an Amino Acid © Allison Calabrese
- Chemical Structure of Alanine © Pierce College is licensed under a CC BY (Attribution) license
- Chemical Structure of Methionine © Pierce College is licensed under a CC BY (Attribution) license
