8.1 Adenosine Triphosphate (ATP)
Figure 8.2 Chemical Structure of ATP
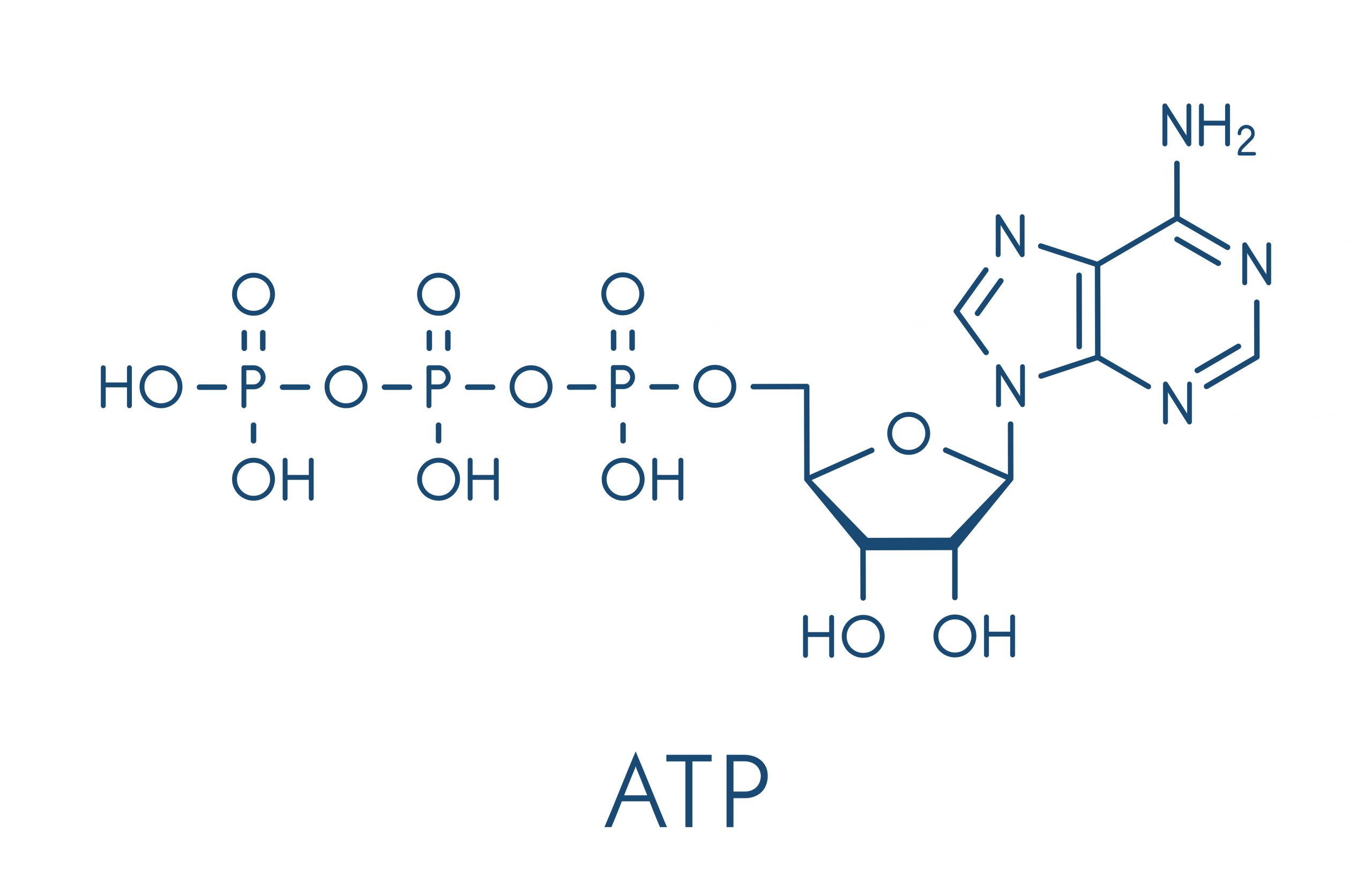
The primary form of chemical energy used in the human body is a chemical compound called adenosine triphosphate (ATP). Structurally, as seen in Figure 8.2, an ATP molecule consists of an adenine (nitrogen containing), a ribose (five carbon sugar), and three phosphate groups.. The chemical bond between the second and third phosphate groups, termed a high-energy bond, represents the greatest source of energy in a cell. It is the first bond that catabolic enzymes break when cells require energy to do work. During this reaction, a molecule of water is added (in a hydrolysis reaction) to the bond between the second and third phosphate groups. The bond is hydrolyzed and the energy is released.
Figure 8.3 Hydrolysis (left side) and Rephosphorylation (right side) of ATP
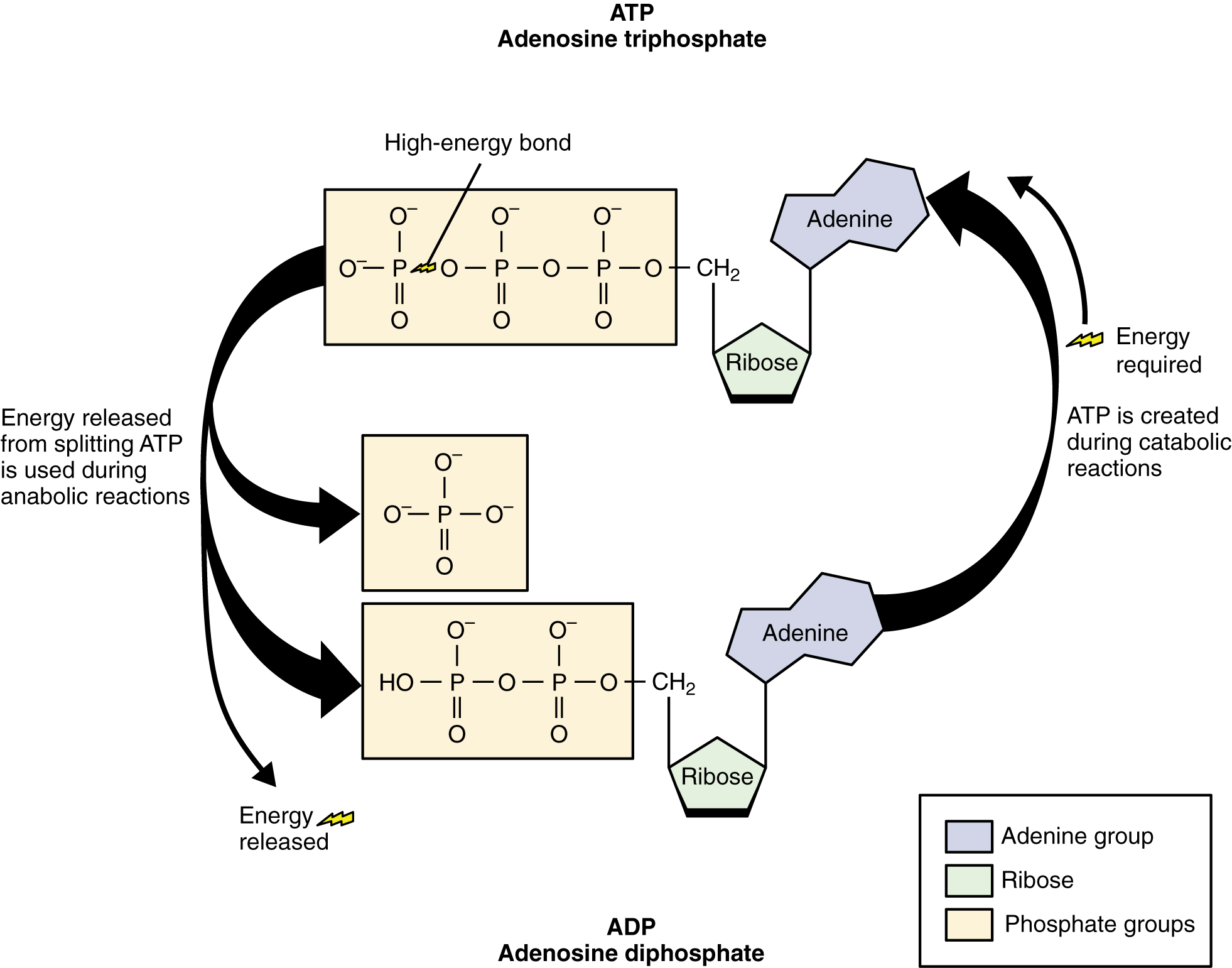
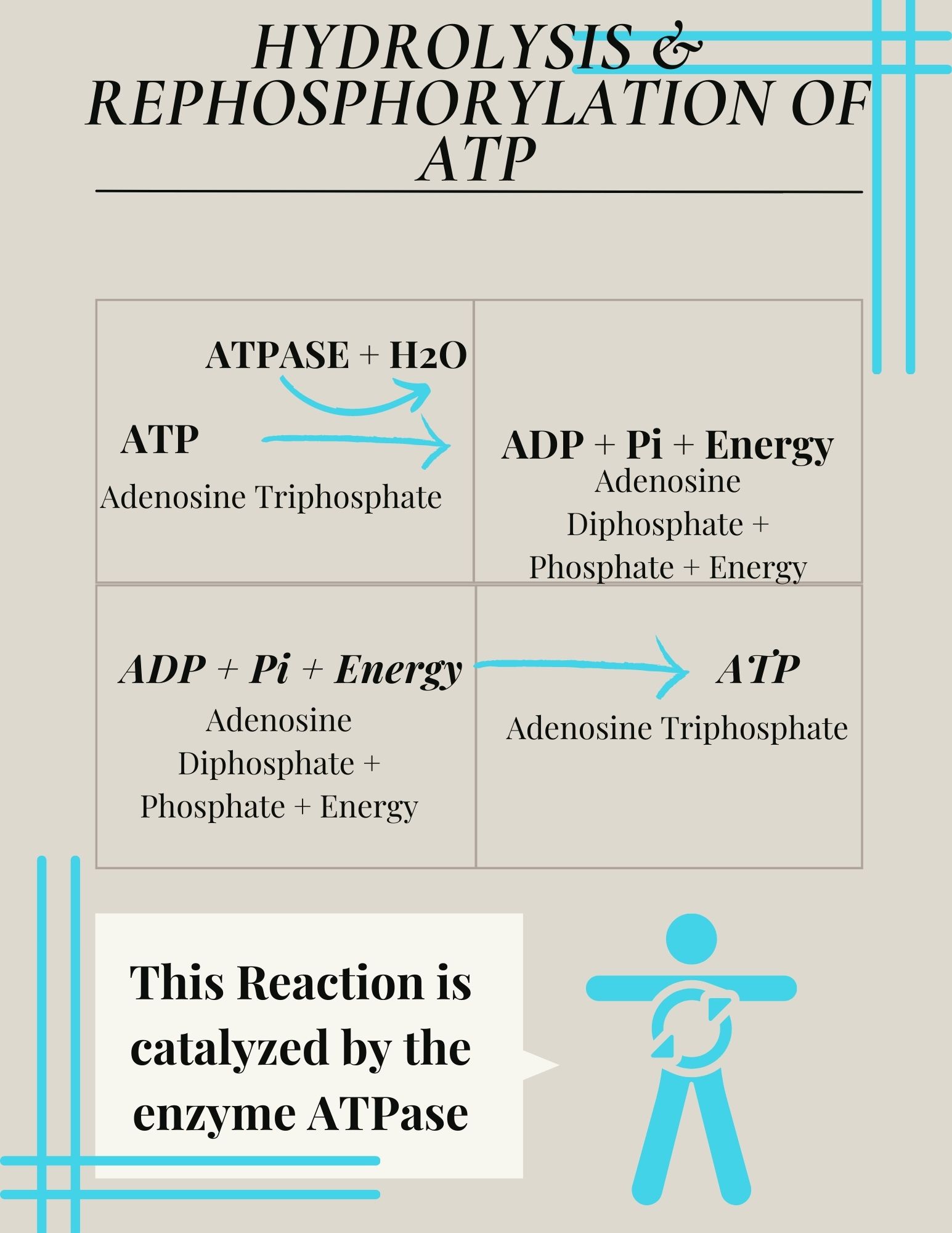
This reaction is catalyzed by the enzyme ATPase and the products of this reaction are a molecule of adenosine diphosphate (ADP) and a lone inorganic phosphate group (Pi). ATP, ADP, and Pi are constantly being cycled through reactions that build ATP and store energy, and reactions that break down ATP and release energy.The energy from ATP drives all bodily functions, such as contracting muscles, maintaining the electrical potential of nerve cells, and absorbing food in the gastrointestinal tract. During exercise, the energy released from this reaction can be harnessed by the exercising muscle and converted to the mechanical energy required for muscle contraction.
ATP can be produced from carbohydrates, fats, amino acids, and creatine phosphate. The body does not store ATP to any great extent. Small amounts are stored in the fibers of skeletal muscles, but only enough to support a few seconds of muscle contraction. After ATP is broken down for energy, it needs to be resynthesized (from ADP and Pi) in order for muscle contraction to continue. The process of resynthesizing ADP back to ATP is called rephosphorylation and can be seen in Figure 8.3. In this reaction, ADP is reconnected to Pi to produce ATP. Creatine phosphate (CP) is another high-energy phosphate compound that is stored in the muscles and is used to rapidly rephosphorylate ADP to ATP when ATP levels decrease. ATP is also made from your body’s stores of carbohydrate, protein, and fat. Carbohydrate and CP can be used to make ATP very quickly, while fat makes ATP more slowly but in greater amounts. Protein can be used to make ATP but it makes ATP slowly and in small amounts so does not provide a significant contribution to your body’s overall energy supply.
Figure 8.4 Recap of ATP Reactions
As mentioned earlier, ATP is not stored to any great extent in the body so we can say that ATP production is a “make it as you go” system. For example, at rest your ATP needs are low, so ATP production is slow but if you need more ATP because you want to go for a run, your body will respond to that demand by increasing the amount of ATP produced. There are three major energy systems, as seen in Figure 8.5, the body can use to make or resynthesize ATP during exercise. These energy systems will be covered in more detail later in this chapter.
Figure 8.5 Energy Systems that Contribute to ATP Production
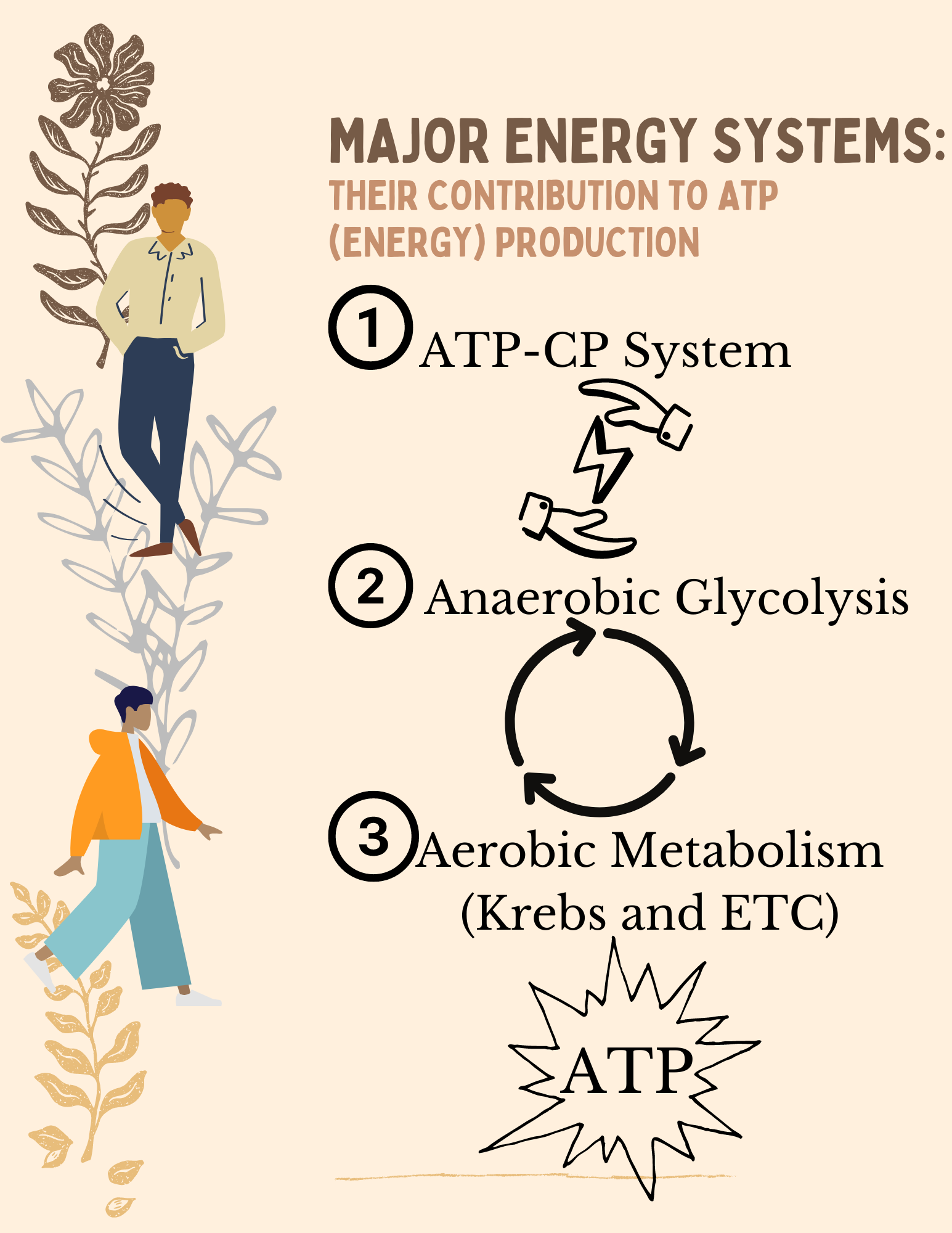
The three primary energy producing systems in the human body are the ATP–CP system (also known as the phosphagen system), anaerobic glycolysis, and aerobic metabolism (Krebs cycle/electron transport system). Before we begin discussing the amount of ATP produced and speed of each of the three systems, it is important to understand how the systems work together to provide the body with a steady supply of energy during different types of activity and as the intensity of an activity changes. Physical activity can be performed at many different intensities and durations. In general, the intensity and the duration of the activity determine which energy system is predominating as the main source of ATP production. The term “predominating” is key, because no energy system operates at the exclusion of the other systems. All three systems operate simultaneously and continuously. One system may predominate as the main producer of ATP, but the other systems are still active, just to a lesser degree. As we discuss the different systems, we are also giving examples of activities that predominantly use that energy system. Always remembering that the other systems are still contributing, even if only a small percentage, to the overall ATP production.
Media Attributions
- Chemical Structure of ATP © Adobe Stock is licensed under a All Rights Reserved license
- Creation of ATP © OpenStax is licensed under a CC BY (Attribution) license
- Hydrolysis & Rephosphorylation of ATP © Natalie Fox is licensed under a CC BY (Attribution) license
- Major Energy Systems and ATP Production © Natalie Fox is licensed under a CC BY (Attribution) license
