9 Early Hominins
Kerryn Warren, Ph.D., Grad Coach International
Lindsay Hunter, M.A., University of Iowa
Navashni Naidoo, M.Sc., University of Cape Town
Silindokuhle Mavuso, M.Sc., University of Witwatersrand
This chapter is a revision from “Chapter 9: Early Hominins” by Kerryn Warren, K. Lindsay Hunter, Navashni Naidoo, Silindokuhle Mavuso, Kimberleigh Tommy, Rosa Moll, and Nomawethu Hlazo. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Understand what is meant by “derived” and “ancestral” traits and why this is relevant for understanding early hominin evolution.
- Understand changing paleoclimates and paleoenvironments as potential factors influencing early hominin adaptations.
- Describe the anatomical changes associated with bipedalism and dentition in early hominins, as well as their implications..
- Describe early hominin genera and species, including their currently understood dates and geographic expanses.
- Describe the earliest stone tool techno-complexes and their impact on the transition from early hominins to our genus.
Defining Hominins
It is through our study of our hominin ancestors and relatives that we are exposed to a world of “might have beens”: of other paths not taken by our species, other ways of being human. But to better understand these different evolutionary trajectories, we must first define the terms we are using. If an imaginary line were drawn between ourselves and our closest relatives, the great apes, bipedalism (or habitually walking upright on two feet) is where that line would be. Hominin, then, means everyone on “our” side of the line: humans and all of our extinct bipedal ancestors and relatives since our divergence from the last common ancestor (LCA) we share with chimpanzees.
Historic interpretations of our evolution, prior to our finding of early hominin fossils, varied. Debates in the mid-1800s regarding hominin origins focused on two key issues:
- Where did we evolve?
- Which traits evolved first?
Charles Darwin hypothesized that we evolved in Africa, as he was convinced that we shared greater commonality with chimpanzees and gorillas on the continent (Darwin 1871). Others, such as Ernst Haeckel and Eugène Dubois, insisted that we were closer in affinity to orangutans and that we evolved in Eurasia where, until the discovery of the Taung Child in South Africa in 1924, all humanlike fossils (of Neanderthals and Homo erectus) had been found (Shipman 2002).
Within this conversation, naturalists and early paleoanthropologists (people who study human evolution) speculated about which human traits came first. These included the evolution of a big brain (encephalization), the evolution of the way in which we move about on two legs (bipedalism), and the evolution of our flat faces and small teeth (indications of dietary change). Original hypotheses suggested that, in order to be motivated to change diet and move about in a bipedal fashion, the large brain needed to have evolved first, as is seen in the fossil species mentioned above.
However, we now know that bipedal locomotion is one of the first things that evolved in our lineage, with early relatives having more apelike dentition and small brain sizes. While brain size expansion is seen primarily in our genus, Homo, earlier hominin brain sizes were highly variable between and within taxa, from 300 cc (cranial capacity, cm3), estimated in Ardipithecus, to 550 cc, estimated in Paranthropus boisei. The lower estimates are well within the range of variation of nonhuman extant great apes. In addition, body size variability also plays a role in the interpretation of whether brain size could be considered large or small for a particular species or specimen. In this chapter, we will tease out the details of early hominin evolution in terms of morphology (i.e. the study of the form, size, or shape of things; in this case, skeletal parts).
We also know that early human evolution occurred in a very complicated fashion. There were multiple species (multiple genera) that featured diversity in their diets and locomotion. Specimens have been found all along the East African Rift System (EARS); that is, in Ethiopia, Kenya, Tanzania, and Malawi; see Figure 9.1), in limestone caves in South Africa, and in Chad. Dates of these early relatives range from around 7 million years ago (mya) to around 1 mya, overlapping temporally with members of our genus, Homo.

Yet there is still so much to understand. Modern debates now look at the relatedness of these species to us and to one another, and they consider which of these species were able to make and use tools. As a result, every site discovery in the patchy hominin fossil record tells us more about our evolution. In addition, recent scientific techniques (not available even ten years ago) provide new insights into the diets, environments, and lifestyles of these ancient relatives.
In the past, taxonomy was primarily based on morphology. Today it is tied to known relationships based on molecular phylogeny (e.g., based on DNA) or a combination of the two. This is complicated when applied to living taxa, but becomes much more difficult when we try to categorize ancestor-descendant relationships for long-extinct species whose molecular information is no longer preserved. We therefore find ourselves falling back on morphological comparisons, often of teeth and partially fossilized skeletal material.
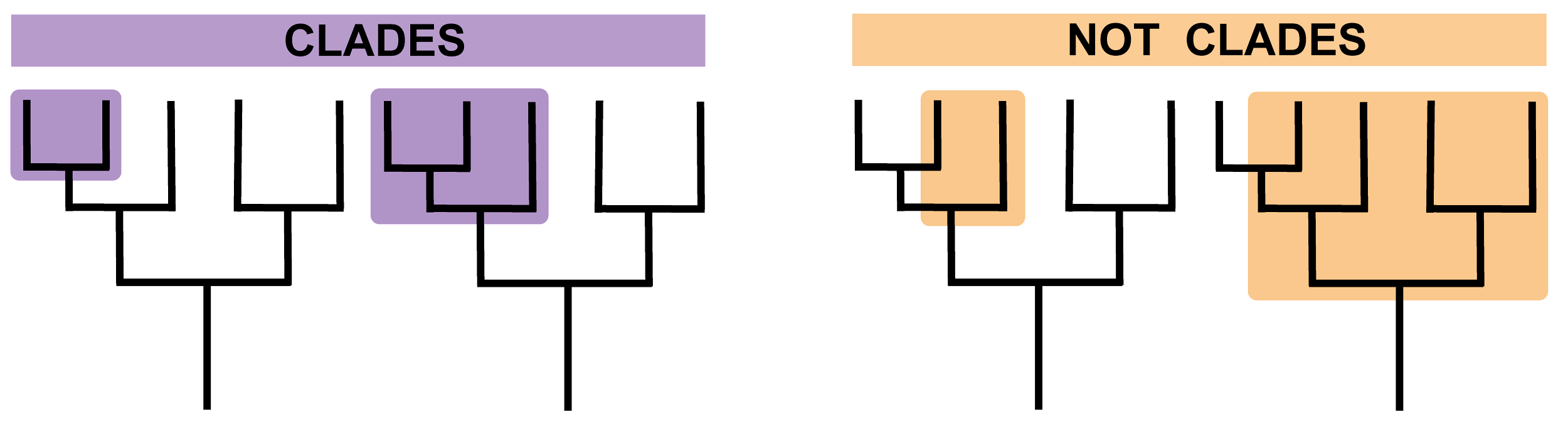
It is here that we turn to the related concepts of cladistics and phylogenetics. Cladistics groups organisms according to their last common ancestors based on shared derived traits. In the case of early hominins, these are often morphological traits that differ from those seen in earlier populations. These new or modified traits provide evidence of evolutionary relationships, and organisms with the same derived traits are grouped in the same clade (Figure 9.2). For example, if we use feathers as a trait, we can group pigeons and ostriches into the clade of birds. In this chapter, we will examine the grouping of the Robust Australopithecines, whose cranial and dental features differ from those of earlier hominins, and therefore are considered derived.
Dig Deeper: Problems Defining Hominin Species
It is worth noting that species designations for early hominin specimens are often highly contested. This is due to the fragmentary nature of the fossil record, the large timescale (millions of years) with which paleoanthropologists need to work, and the difficulty in evaluating whether morphological differences and similarities are due to meaningful phylogenetic or biological differences or subtle differences/variation in niche occupation or time. In other words, do morphological differences really indicate different species? How would classifying species in the paleoanthropological record compare with classifying living species today, for whom we can sequence genomes and observe lifestyles?
There are also broader philosophical differences among researchers when it comes to paleo-species designations. Some scientists, known as “lumpers,” argue that large variability is expected among multiple populations in a given species over time. These researchers will therefore prefer to “lump” specimens of subtle differences into single taxa. Others, known as “splitters,” argue that species variability can be measured and that even subtle differences can imply differences in niche occupation that are extreme enough to mirror modern species differences. In general, splitters would consider geographic differences among populations as meaning that a species is polytypic (i.e., capable of interacting and breeding biologically but having morphological population differences). This is worth keeping in mind when learning about why species designations may be contested.
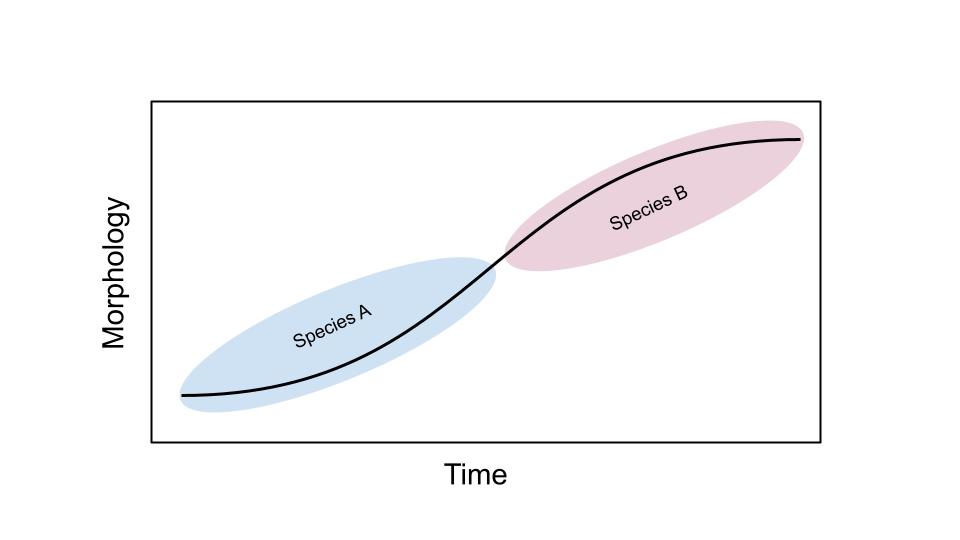
This further plays a role in evaluating ancestry. Debates over which species “gave rise” to which continue to this day. It is common to try to create “lineages” of species to determine when one species evolved into another over time. We refer to these as chronospecies (Figure 9.3). Constructed hominin phylogenetic trees are routinely variable, changing with new specimen discoveries, new techniques for evaluating and comparing species, and, some have argued, nationalist or biased interpretations of the record. More recently, some researchers have shifted away from “treelike” models of ancestry toward more nuanced metaphors such as the “braided stream,” where some levels of interbreeding among species and populations are seen as natural processes of evolution.
Finally, it is worth considering the process of fossil discovery and publication. Some fossils are easily diagnostic to a species level and allow for easy and accurate interpretation. Some, however, are more controversial. This could be because they do not easily preserve or are incomplete, making it difficult to compare and place within a specific species (e.g., a fossil of a patella or knee bone). Researchers often need to make several important claims when announcing or publishing a find: a secure date (if possible), clear association with other finds, and an adequate comparison among multiple species (both extant and fossil). Therefore, it is not uncommon that an important find was made years before it is scientifically published.
Paleoenvironment and Hominin Evolution
There is no doubt that one of the major selective pressures in hominin evolution is the environment. Large-scale changes in global and regional climate, as well as alterations to the environment, are all linked to hominin diversification, dispersal, and extinction (Maslin et al. 2014). Environmental reconstructions often use modern analogues. Let us take, for instance, the hippopotamus. It is an animal that thrives in environments that have abundant water to keep its skin cool and moist. If the environment for some reason becomes drier, it is expected that hippopotamus populations will reduce. If a drier environment becomes wetter, it is possible that hippopotamus populations may be attracted to the new environment and thrive. Such instances have occurred multiple times in the past, and the bones of some fauna (i.e., animals, like the hippopotamus) that are sensitive to these changes give us insights into these events.
Yet reconstructing a paleoenvironment relies on a range of techniques, which vary depending on whether research interests focus on local changes or more global environmental changes/reconstructions. For local environments (such as a single site or region), comparing the faunal assemblages (collections of fossils of animals found at a site) with animals found in certain modern environments allows us to determine if past environments mirror current ones in the region. Changes in the faunal assemblages, as well as when they occur and how they occur, tell us about past environmental changes. Other techniques are also useful in this regard. Chemical analyses, for instance, can reveal the diets of individual fauna, providing clues as to the relative wetness or dryness of their environment (e.g., nitrogen isotopes; Kingston and Harrison 2007).
Global climatic changes in the distant past, which fluctuated between being colder and drier and warmer and wetter on average, would have global implications for environmental change (Figure 9.4). These can be studied by comparing marine core and terrestrial soil data across multiple sites. These techniques are based on chemical analysis, such as examination of the nitrogen and oxygen isotopes in shells and sediments. Similarly, analyzing pollen grains shows which kinds of flora survived in an environment at a specific time period. There are multiple lines of evidence that allow us to visualize global climate trends over millions of years (although it should be noted that the direction and extent of these changes could differ by geographic region).
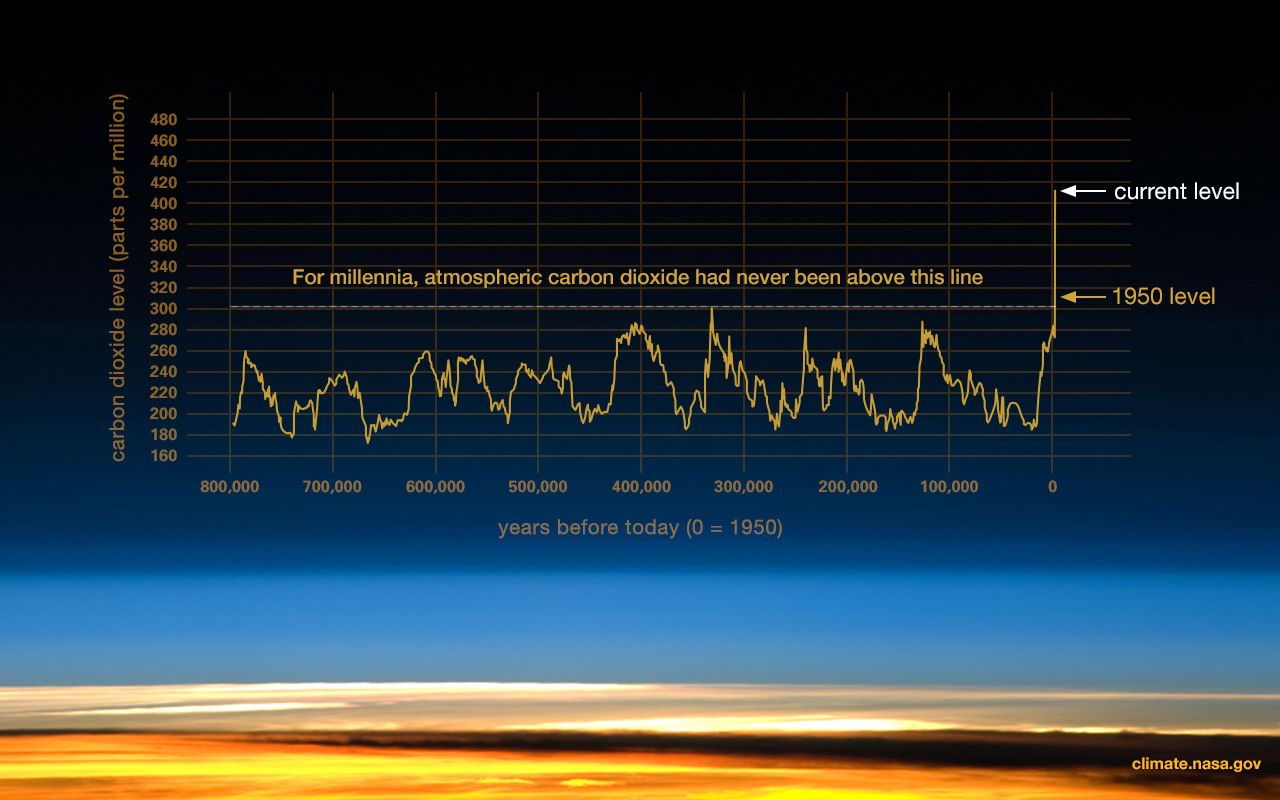
Both local and global climatic/environmental changes have been used to understand factors affecting our evolution (DeHeinzelin et al. 1999; Kingston 2007). Environmental change acts as an important factor regarding the onset of several important hominin traits seen in early hominins and discussed in this chapter. Namely, the environment has been interpreted as the following:
- the driving force behind the evolution of bipedalism,
- the reason for change and variation in early hominin diets, and
- the diversification of multiple early hominin species.
There are numerous hypotheses regarding how climate has driven and continues to drive human evolution. Here, we will focus on just three popular hypotheses.
Savannah Hypothesis (or Aridity Hypothesis)
The hypothesis: This popular theory suggests that the expansion of the savannah (or less densely forested, drier environments) forced early hominins from an arboreal lifestyle (one living in trees) to a terrestrial one where bipedalism was a more efficient form of locomotion (Figure 9.5). It was first proposed by Darwin (1871) and supported by anthropologists like Raymond Dart (1925). However, this idea was supported by little fossil or paleoenvironmental evidence and was later refined as the Aridity Hypothesis. This hypothesis states that the long-term aridification and, thereby, expansion of savannah biomes were drivers in diversification in early hominin evolution (deMenocal 2004; deMenocal and Bloemendal 1995). It advocates for periods of accelerated aridification leading to early hominin speciation events.

The evidence: While early bipedal hominins are often associated with wetter, more closed environments (i.e., not the Savannah Hypothesis), both marine and terrestrial records seem to support general cooling, drying conditions, with isotopic records indicating an increase in grasslands (i.e., colder and wetter climatic conditions) between 8 mya and 6 mya across the African continent (Cerling et al. 2011). This can be contrasted with later climatic changes derived from aeolian dust records (sediments transported to the site of interest by wind), which demonstrate increases in seasonal rainfall between 3 mya and 2.6 mya, 1.8 mya and 1.6 mya, and 1.2 mya and 0.8 mya (deMenocal 2004; deMenocal and Bloemendal 1995).
Interpretation(s): Despite a relatively scarce early hominin record, it is clear that two important factors occur around the time period in which we see increasing aridity. The first factor is the diversification of taxa, where high morphological variation between specimens has led to the naming of multiple hominin genera and species. The second factor is the observation that the earliest hominin fossils appear to have traits associated with bipedalism and are dated to around the drying period (as based on isotopic records). Some have argued that it is more accurately a combination of bipedalism and arboreal locomotion, which will be discussed later. However, the local environments in which these early specimens are found (as based on the faunal assemblages) do not appear to have been dry.
Turnover Pulse Hypothesis
The hypothesis: In 1985, paleontologist Elisabeth Vbra noticed that in periods of extreme and rapid climate change, ungulates (hoofed mammals of various kinds) that had generalized diets fared better than those with specialized diets (Vrba 1988, 1998). Specialist eaters (those who rely primarily on specific food types) faced extinction at greater rates than their generalist (those who can eat more varied and variable diets) counterparts because they were unable to adapt to new environments (Vrba 2000). Thus, periods with extreme climate change would be associated with high faunal turnover: that is, the extinction of many species and the speciation, diversification, and migration of many others to occupy various niches.
The evidence: The onset of the Quaternary Ice Age, between 2.5 mya and 3 mya, brought extreme global, cyclical interglacial and glacial periods (warmer, wetter periods with less ice at the poles, and colder, drier periods with more ice near the poles). Faunal evidence from the Turkana basin in East Africa indicates multiple instances of faunal turnover and extinction events, in which global climatic change resulted in changes from closed/forested to open/grassier habitats at single sites (Behrensmeyer et al. 1997; Bobe and Behrensmeyer 2004). Similarly, work in the Cape Floristic Belt of South Africa shows that extreme changes in climate play a role in extinction and migration in ungulates. While this theory was originally developed for ungulates, its proponents have argued that it can be applied to hominins as well. However, the link between climate and speciation is only vaguely understood (Faith and Behrensmeyer 2013).
Interpretation(s): While the evidence of rapid faunal turnover among ungulates during this time period appears clear, there is still some debate around its usefulness as applied to the paleoanthropological record. Specialist hominin species do appear to exist for long periods of time during this time period, yet it is also true that Homo, a generalist genus with a varied and adaptable diet, ultimately survives the majority of these fluctuations, and the specialists appear to go extinct.
Variability Selection Hypothesis
The hypothesis: This hypothesis was first articulated by paleoanthropologist Richard Potts (1998). It links the high amount of climatic variability over the last 7 million years to both behavioral and morphological changes. Unlike previous notions, this hypothesis states that hominin evolution does not respond to habitat-specific changes or to specific aridity or moisture trends. Instead, long-term environmental unpredictability over time and space influenced morphological and behavioral adaptations that would help hominins survive, regardless of environmental context (Potts 1998, 2013). The Variability Selection Hypothesis states that hominin groups would experience varying degrees of natural selection due to continually changing environments and potential group isolation. This would allow certain groups to develop genetic combinations that would increase their ability to survive in shifting environments. These populations would then have a genetic advantage over others that were forced into habitat-specific adaptations (Potts 2013).
The evidence: The evidence for this theory is similar to that for the Turnover Pulse Hypothesis: large climatic variability and higher survivability of generalists versus specialists. However, this hypothesis accommodates for larger time-scales of extinction and survival events.
Interpretation(s): In this way, the Variability Selection Hypothesis allows for a more flexible interpretation of the evolution of bipedalism in hominins and a more fluid interpretation of the Turnover Pulse Hypothesis, where species turnover is meant to be more rapid. In some ways, this hypothesis accommodates both environmental data and our interpretations of an evolution toward greater variability among species and the survivability of generalists.
Paleoenvironment Summary
Some hypotheses presented in this section pay specific attention to habitat (Savannah Hypothesis) while others point to large-scale climatic forces (Variability Selection Hypothesis). Some may be interpreted to describe the evolution of traits such as bipedalism (Savannah Hypothesis), and others generally explain the diversification of early hominins (Turnover Pulse and Variability Selection Hypotheses). While there is no consensus as to how the environment drove our evolution, it is clear that the environment shaped both habitat and resource availability in ways that would have influenced our early ancestors physically and behaviorally.
Derived Adaptations: Bipedalism
The unique form of locomotion exhibited by modern humans, called obligate bipedalism, is important in distinguishing our species from the extant (living) great apes. The ability to walk habitually upright is thus considered one of the defining attributes of the hominin lineage. We also differ from other animals that walk bipedally (such as kangaroos) in that we do not have a tail to balance us as we move.
The origin of bipedalism in hominins has been debated in paleoanthropology, but at present there are two main ideas:
- early hominins initially lived in trees, but increasingly started living on the ground, so we were a product of an arboreal last common ancestor (LCA) or,
- our LCA was a terrestrial quadrupedal knuckle-walking species, more similar to extant chimpanzees.
Most research supports the first theory of an arboreal LCA based on skeletal morphology of early hominin genera that demonstrate adaptations for climbing but not for knuckle-walking. This would mean that both humans and chimpanzees can be considered “derived” in terms of locomotion since chimpanzees would have independently evolved knuckle-walking.
There are many current ideas regarding selective pressures that would lead to early hominins adapting upright posture and locomotion. Many of these selective pressures, as we have seen in the previous section, coincide with a shift in environmental conditions, supported by paleoenvironmental data. In general, however, it appears that, like extant great apes, early hominins thrived in forested regions with dense tree coverage, which would indicate an arboreal lifestyle. As the environmental conditions changed and a savannah/grassland environment became more widespread, the tree cover would become less dense, scattered, and sparse such that bipedalism would become more important.
There are several proposed selective pressures for bipedalism:
- Energy conservation: Modern bipedal humans conserve more energy than extant chimpanzees, which are predominantly knuckle-walking quadrupeds when walking over land. While chimpanzees, for instance, are faster than humans terrestrially, they expend large amounts of energy being so. Adaptations to bipedalism include “stacking” the majority of the weight of the body over a small area around the center of gravity (i.e., the head is above the chest, which is above the pelvis, which is over the knees, which are above the feet). This reduces the amount of muscle needed to be engaged during locomotion to “pull us up” and allows us to travel longer distances expending far less energy.
- Thermoregulation: Less surface area (i.e., only the head and shoulders) is exposed to direct sunlight during the hottest parts of the day (i.e., midday). This means that the body has less need to employ additional “cooling” mechanisms such as sweating, which additionally means less water loss.
- Bipedalism: This method of locomotion freed up our ancestors’ hands such that they could more easily gather food and carry tools or infants. This further enabled the use of hands for more specialized adaptations associated with the manufacturing and use of tools.
These selective pressures are not mutually exclusive. Bipedality could have evolved from a combination of these selective pressures, in ways that increased the chances of early hominin survival.
Skeletal Adaptations for Bipedalism
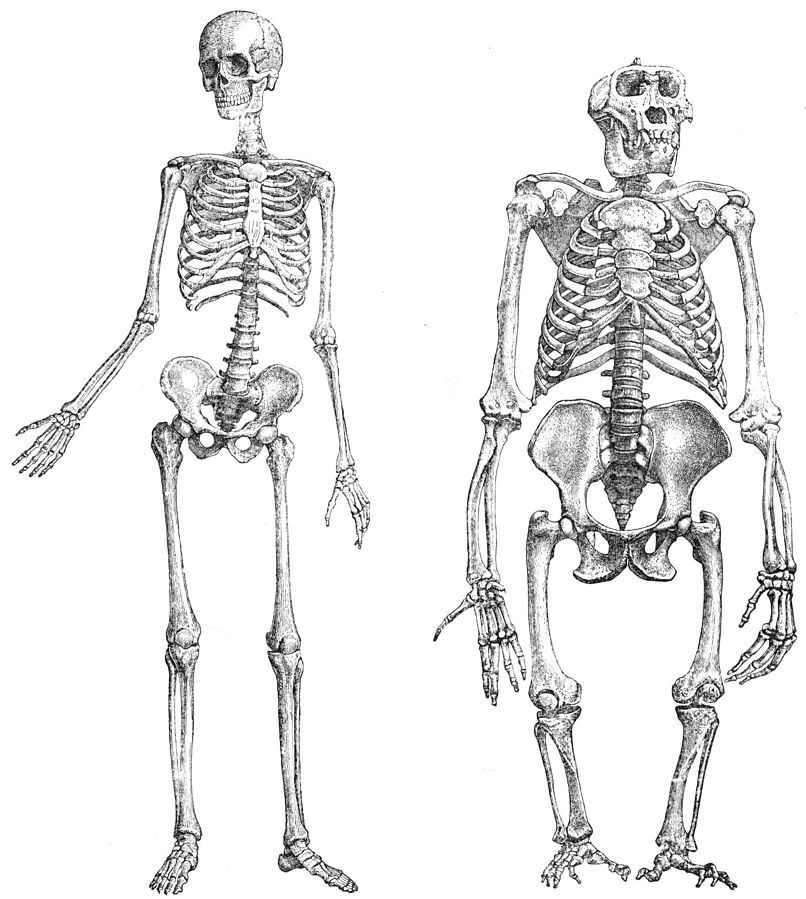
Humans have highly specialized adaptations to facilitate obligate bipedalism (Figure 9.6). Many of these adaptations occur within the soft tissue of the body (e.g., muscles and tendons). However, when analyzing the paleoanthropological record for evidence of the emergence of bipedalism, all that remains is the fossilized bone. Interpretations of locomotion are therefore often based on comparative analyses between fossil remains and the skeletons of extant primates with known locomotor behaviors. These adaptations occur throughout the skeleton and are summarized in Figure 9.7.
The majority of these adaptations occur in the postcranium (the skeleton from below the head) and are outlined in Figure 9.7. In general, these adaptations allow for greater stability and strength in the lower limb, by allowing for more shock absorption, for a larger surface area for muscle attachment, and for the “stacking” of the skeleton directly over the center of gravity to reduce energy needed to be kept upright. These adaptations often mean less flexibility in areas such as the knee and foot.
However, these adaptations come at a cost. Evolving from a nonobligate bipedal ancestor means that the adaptations we have are evolutionary compromises. For instance, the valgus knee (angle at the knee) is an essential adaptation to balance the body weight above the ankle during bipedal locomotion. However, the strain and shock absorption at an angled knee eventually takes its toll. For example, runners often experience joint pain. Similarly, the long neck of the femur absorbs stress and accommodates for a larger pelvis, but it is a weak point, resulting in hip replacements being commonplace among the elderly, especially in cases where the bone additionally weakens through osteoporosis. Finally, the S-shaped curve in our spine allows us to stand upright, relative to the more curved C-shaped spine of an LCA. Yet the weaknesses in the curves can lead to pinching of nerves and back pain. Since many of these problems primarily are only seen in old age, they can potentially be seen as an evolutionary compromise.
Despite relatively few postcranial fragments, the fossil record in early hominins indicates a complex pattern of emergence of bipedalism. Key features, such as a more anteriorly placed foramen magnum, are argued to be seen even in the earliest discovered hominins, indicating an upright posture (Dart 1925). Some early species appear to have a mix of ancestral (arboreal) and derived (bipedal) traits, which indicates a mixed locomotion and a more mosaic evolution of the trait. Some early hominins appear to, for instance, have bowl-shaped pelvises (hip bones) and angled femurs suitable for bipedalism but also have retained an opposable hallux (big toe) or curved fingers and longer arms (for arboreal locomotion). These mixed morphologies may indicate that earlier hominins were not fully obligate bipeds and thus thrived in mosaic environments.
Yet the associations between postcranial and the more diagnostic cranial fossils and bones are not always clear, muddying our understanding of the specific species to which fossils belong (Grine et al. 2022).
| Region | Feature | Obligate Biped (H. sapiens) | Nonobligate Biped |
|---|---|---|---|
| Cranium | Position of the foramen magnum | Positioned inferiorly (immediately under the cranium) so that the head rests on top of the vertebral column for balance and support (head is perpendicular to the ground). | Posteriorly positioned (to the back of the cranium). Head is positioned parallel to the ground. |
| Post
cranium |
Body proportions | Shorter upper limb (not used for locomotion). | Longer upper limbs (used for locomotion). |
| Post
cranium |
Spinal curvature | S-curve due to pressure exerted on the spine from bipedalism (lumbar lordosis). | C-curve. |
| Post
cranium |
Vertebrae | Robust lumbar (lower-back) vertebrae (for shock absorbance and weight bearing). Lower back is more flexible than that of apes as the hips and trunk swivel when walking (weight transmission). | Gracile lumbar vertebrae compared to those of modern humans. |
| Post
cranium |
Pelvis | Shorter, broader, bowl-shaped pelvis (for support); very robust. Broad sacrum with large sacroiliac joint surfaces. | Longer, flatter, elongated ilia; more narrow and gracile; narrower sacrum; relatively smaller sacroiliac joint surface. |
| Post
cranium |
Lower limb | In general, longer, more robust lower limbs and more stable, larger joints.
|
In general, smaller, more gracile limbs with more flexible joints.
|
| Post
cranium |
Foot | Rigid, robust foot, without a midtarsal break.
Nonopposable and large, robust big toe (for push off while walking) and large heel for shock absorbance. |
Flexible foot, midtarsal break present (which allows primates to lift their heels independently from their feet), opposable big toe for grasping. |
It is also worth noting that, while not directly related to bipedalism per se, other postcranial adaptations are evident in the hominin fossil record from some of the earlier hominins. For instance, the hand and finger morphologies of many of the earliest hominins indicate adaptations consistent with arboreality. These include longer hands, more curved metacarpals and phalanges (long bones in the hand and fingers, respectively), and a shorter, relatively weaker thumb. This allows for gripping onto curved surfaces during locomotion. The earliest hominins appear to have mixed morphologies for both bipedalism and arborealism. However, among Australopiths (members of the genus, Australopithecus), there are indications for greater reliance on bipedalism as the primary form of locomotion. Similarly, adaptations consistent with tool manufacture (shorter fingers and a longer, more robust thumb, in contrast to the features associated with arborealism) have been argued to appear before the genus Homo.
Early Hominins: Sahelanthropus and Orrorin
We see evidence for bipedalism in some of the earliest fossil hominins, dated from within our estimates of our divergence from chimpanzees. These hominins, however, also indicate evidence for arboreal locomotion.
The earliest dated hominin find (between 6 mya and 7 mya, based on radiometric dating of volcanic tufts) has been argued to come from Chad and is named Sahelanthropus tchadensis (Figure 9.8; Brunet et al. 1995). The initial discovery was made in 2001 by Ahounta Djimdoumalbaye and announced in Nature in 2002 by a team led by French paleontologist Michel Brunet. The find has a small cranial capacity (360 cc) and smaller canines than those in extant great apes, though they are larger and pointier than those in humans. This implies strongly that, over evolutionary time, the need for display and dominance among males has reduced, as has our sexual dimorphism. A short cranial base and a foramen magnum that is more humanlike in positioning have been argued to indicate upright walking.
Initially, the inclusion of Sahelanthropus in the hominin family was debated by researchers, since the evidence for bipedalism is based on cranial evidence alone, which is not as convincing as postcranial evidence. Yet, a femur (thigh bone) and ulnae (upper arm bones) thought to belong to Sahelanthropus was discovered in 2001 (although not published until 2022). These bones may support the idea that the hominin was in fact a terrestrial biped with arboreal capabilities and behaviors (Daver et al. 2022).
Orrorin tugenensis (Orrorin meaning “original man”), dated to between 6 mya and 5.7 mya, was discovered near Tugen Hills in Kenya in 2000. Smaller cheek teeth (molars and premolars) than those in even more recent hominins, thick enamel, and reduced, but apelike, canines characterize this species. This is the first species that clearly indicates adaptations for bipedal locomotion, with fragmentary leg, arm, and finger bones having been found but few cranial remains. One of the most important elements discovered was a proximal femur, BAR 1002’00. The femur is the thigh bone, and the proximal part is that which articulates with the pelvis; this is very important for studying posture and locomotion. This femur indicates that Ororrin was bipedal, and recent studies suggest that it walked in a similar way to later Pliocene hominins. Some have argued that features of the finger bones suggest potential tool-making capabilities, although many researchers argue that these features are also consistent with climbing.
Early Hominins: The Genus Ardipithecus
Another genus, Ardipithecus, is argued to be represented by at least two species: Ardipithecus (Ar.) ramidus and Ar. kadabba.
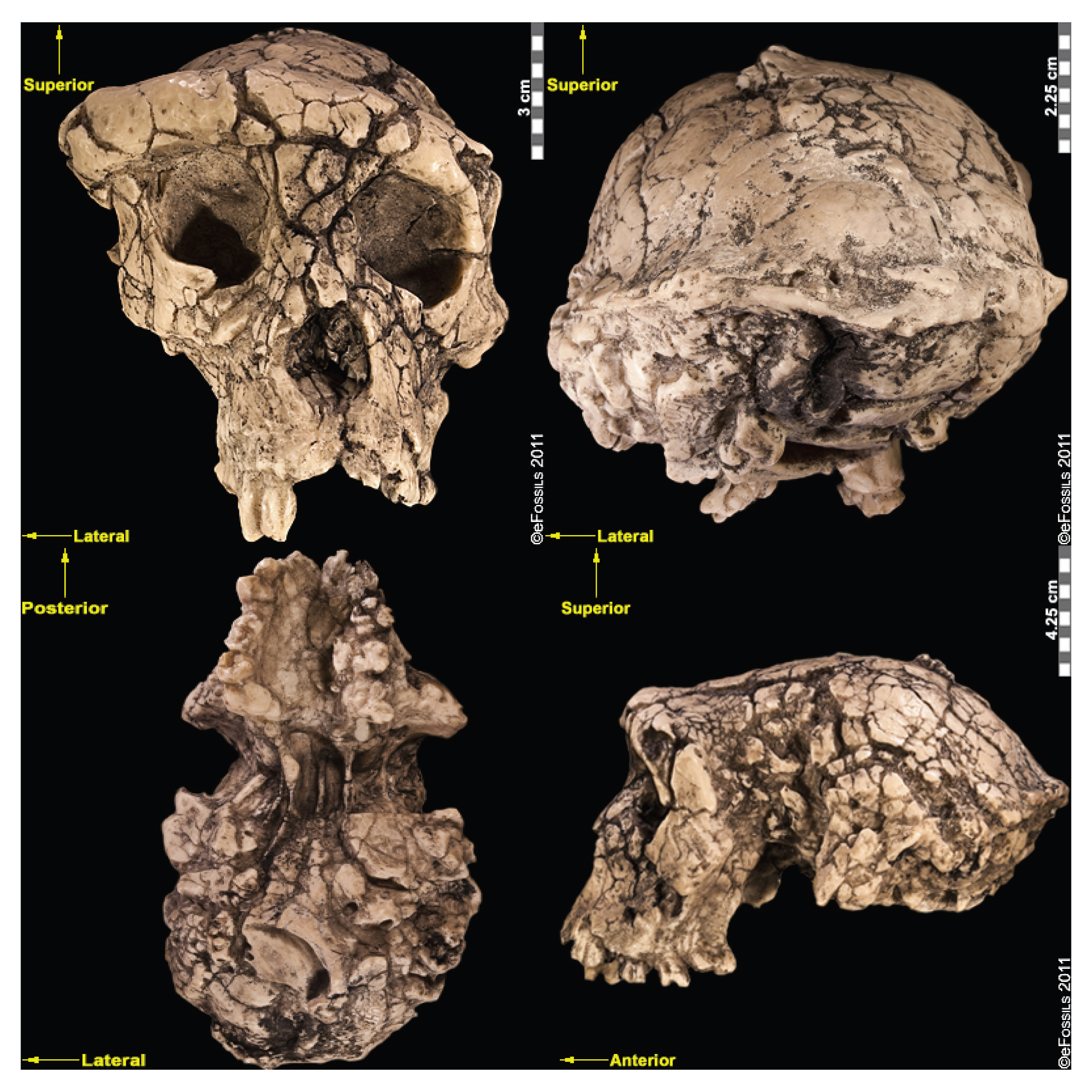
Ardipithecus ramidus (“ramid” means root in the Afar language) is currently the best-known of the earliest hominins (Figure 9.9). Unlike Sahelanthropus and Orrorin, this species has a large sample size of over 110 specimens from Aramis alone. Dated to 4.4 mya, Ar. ramidus was found in Ethiopia (in the Middle Awash region and in Gona). This species was announced in 1994 by American palaeoanthropologist Tim White, based on a partial female skeleton nicknamed “Ardi” (ARA-VP-6/500; White et al. 1994). Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, she had an opposable big toe (hallux), similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. A small brain (300 cc to 350 cc), midfacial projection, and slight prognathism show retained ancestral cranial features, but the cheek bones are less flared and robust than in later hominins.
Ardipithecus kadabba (the species name means “oldest ancestor” in the Afar language) is known from localities on the western margin of the Middle Awash region, the same locality where Ar. ramidus has been found. Specimens include mandibular fragments and isolated teeth as well as a few postcranial elements from the Asa Koma (5.5 mya to 5.77 mya) and Kuseralee Members (5.2 mya), well-dated and understood (but temporally separate) volcanic layers in East Africa. This species was discovered in 1997 by paleoanthropologist Dr. Yohannes Haile-Selassie. Originally these specimens were referred to as a subspecies of Ar. ramidus. In 2002, six teeth were discovered at Asa Koma and the dental-wear patterns confirmed that this was a distinct species, named Ar. kadabba, in 2004. One of the postcranial remains recovered included a 5.2 million-year-old toe bone that demonstrated features that are associated with toeing off (pushing off the ground with the big toe leaving last) during walking, a characteristic unique to bipedal walkers. However, the toe bone was found in the Kuseralee Member, and therefore some doubt has been cast by researchers about its association with the teeth from the Asa Koma Member.
Bipedal Trends in Early Hominins: Summary
Trends toward bipedalism are seen in our earliest hominin finds. However, many specimens also indicate retained capabilities for climbing. Trends include a larger, more robust hallux; a more compact foot, with an arch; a robust, long femur, angled at the knee; a robust tibia; a bowl-shaped pelvis; and a more anterior foramen magnum. While the level of bipedality in Salehanthropus tchadenisis is debated since there are few fossils and no postcranial evidence, Orrorin tugenensis and Ardipithecus kadabba show clear indications of some of these bipedal trends. However, some retained ancestral traits, such as an opposable hallux in Ardipithecus, indicate some retention in climbing ability.
Derived Adaptations: Early Hominin Dentition
The Importance of Teeth
Teeth are abundant in the fossil record, primarily because they are already highly mineralized as they are forming, far more so than even bone. Because of this, teeth preserve readily. And, because they preserve readily, they are well-studied and better understood than many skeletal elements. In the sparse hominin (and primate) fossil record, teeth are, in some cases, all we have.
Teeth also reveal a lot about the individual from whom they came. We can tell what they evolved to eat, to which other species they may be closely related, and even, to some extent, the level of sexual dimorphism, or general variability, within a given species. This is powerful information that can be contained in a single tooth. With a little more observation, the wearing patterns on a tooth can tell us about the diet of the individual in the weeks leading up to its death. Furthermore, the way in which a tooth is formed, and the timing of formation, can reveal information about changes in diet (or even mobility) over infancy and childhood, using isotopic analyses. When it comes to our earliest hominin relatives, this information is vital for understanding how they lived.
The purpose of comparing different hominin species is to better understand the functional morphology as it applies to dentition. In this, we mean that the morphology of the teeth or masticatory system (which includes jaws) can reveal something about the way in which they were used and, therefore, the kinds of foods these hominins ate. When comparing the features of hominin groups, it is worth considering modern analogues (i.e., animals with which to compare) to make more appropriate assumptions about diet. In this way, hominin dentition is often compared with that of chimpanzees and gorillas (our close ape relatives), as well as with that of modern humans.
The most divergent group, however, is humans. Humans around the world have incredibly varied diets. Among hunter-gatherers, it can vary from a honey- and plant-rich diet, as seen in the Hadza in Tanzania, to a diet almost entirely reliant on animal fat and protein, as seen in Inuits in polar regions of the world. We are therefore considered generalists, more general than the largely frugivorous (fruit-eating) chimpanzee or the folivorous (foliage-eating) gorilla, as discussed in Chapter 5.
One way in which all humans are similar is our reliance on the processing of our food. We cut up and tear meat with tools using our hands, instead of using our front teeth (incisors and canines). We smash and grind up hard seeds, instead of crushing them with our hind teeth (molars). This means that, unlike our ape relatives, we can rely more on developing tools to navigate our complex and varied diets. Our brain, therefore, is our primary masticatory organ. Evolutionarily, our teeth have reduced in size and our faces are flatter, or more orthognathic, partially in response to our increased reliance on our hands and brain to process food. Similarly, a reduction in teeth and a more generalist dental morphology could also indicate an increase in softer and more variable foods, such as the inclusion of more meat. These trends begin early on in our evolution. The link has been made between some of the earliest evidence for stone tool manufacture, the earliest members of our genus, and the features that we associate with these specimens.
General Dental Trends in Early Hominins
Several trends are visible in the dentition of early hominins. However, all tend to have the same dental formula. The dental formula tells us how many of each tooth type are present in each quadrant of the mouth. Going from the front of the mouth, this includes the square, flat incisors; the pointy canines; the small, flatter premolars; and the larger hind molars. In many primates, from Old World monkeys to great apes, the typical dental formula is 2:1:2:3. This means that if we divide the mouth into quadrants, each has two incisors, one canine, two premolars, and three molars. The eight teeth per quadrant total 32 teeth in all (although some humans have fewer teeth due to the absence of their wisdom teeth, or third molars).

The morphology of the individual teeth is where we see the most change. Among primates, large incisors are associated with food procurement or preparation (such as biting small fruits), while small incisors indicate a diet that may contain small seeds or leaves (where the preparation is primarily in the back of the mouth). Most hominins have relatively large, flat, vertically aligned incisors that occlude (touch) relatively well, forming a “bite.” This differs from, for instance, the orangutan, whose teeth stick out (i.e., are procumbent).
While the teeth are often aligned with diet, the canines may be misleading in that regard. We tend to associate pointy, large canines with the ripping required for meat, and the reduction (or, in some animals, the absence) of canines as indicative of herbivorous diets. In humans, our canines are often a similar size to our incisors and therefore considered incisiform (Figure 9.10). However, our closest relatives all have very long, pointy canines, particularly on their upper dentition. This is true even for the gorilla, which lives almost exclusively on plants. The canines in these instances reveal more about social structure and sexual dimorphism than diet, as large canines often signal dominance.
Early on in human evolution, we see a reduction in canine size. Sahelanthropus tchadensis and Orrorin tugenensis both have smaller canines than those in extant great apes, yet the canines are still larger and pointier than those in humans or more recent hominins. This implies strongly that, over evolutionary time, the need for display and dominance among males has reduced, as has our sexual dimorphism. In Ardipithecus ramidus, there is no obvious difference between male and female canine size, yet they are still slightly larger and pointier than in modern humans. This implies a less sexually dimorphic social structure in the earlier hominins relative to modern-day chimpanzees and gorillas.
Along with a reduction in canine size is the reduction or elimination of a canine diastema: a gap between the teeth on the mandible that allows room for elongated teeth on the maxilla to “fit” in the mouth. Absence of a diastema is an excellent indication of a reduction in canine size. In animals with large canines (such as baboons), there is also often a honing P3, where the first premolar (also known as P3 for evolutionary reasons) is triangular in shape, “sharpened” by the extended canine from the upper dentition. This is also seen in some early hominins: Ardipithecus, for example, has small canines that are almost the same height as its incisors, although still larger than those in recent hominins.
The hind dentition, such as the bicuspid (two cusped) premolars or the much larger molars, are also highly indicative of a generalist diet in hominins. Among the earliest hominins, the molars are larger than we see in our genus, increasing in size to the back of the mouth and angled in such a way from the much smaller anterior dentition as to give these hominins a parabolic (V-shaped) dental arch. This differs from our living relatives and some early hominins, such as Sahelanthropus, whose molars and premolars are relatively parallel between the left and right sides of the mouth, creating a U-shape.
Among more recent early hominins, the molars are larger than those in the earliest hominins and far larger than those in our own genus, Homo. Large, short molars with thick enamel allowed our early cousins to grind fibrous, coarse foods, such as sedges, which require plenty of chewing. This is further evidenced in the low cusps, or ridges, on the teeth, which are ideal for chewing. In our genus, the hind dentition is far smaller than in these early hominins. Our teeth also have medium-size cusps, which allow for both efficient grinding and tearing/shearing meats.
Understanding the dental morphology has allowed researchers to extrapolate very specific behaviors of early hominins. It is worth noting that while teeth preserve well and are abundant, a slew of other morphological traits additionally provide evidence for many of these hypotheses. Yet there are some traits that are ambiguous. For instance, while there are definitely high levels of sexual dimorphism in Au. afarensis, discussed in the next section, the canine teeth are reduced in size, implying that while canines may be useful indicators for sexual dimorphism, it is also worth considering other evidence.
In summary, trends among early hominins include a reduction in procumbency, reduced hind dentition (molars and premolars), a reduction in canine size (more incisiform with a lack of canine diastema and honing P3), flatter molar cusps, and thicker dental enamel. All early hominins have the ancestral dental formula of 2:1:2:3. These trends are all consistent with a generalist diet, incorporating more fibrous foods.
Special Topic: Contested Species
Many named species are highly debated and argued to have specimens associated with a more variable Au. afarensis or Au. anamensis species. Sometimes these specimens are dated to times when, or found in places in which, there are “gaps” in the palaeoanthropological record. These are argued to represent chronospecies or variants of Au. afarensis. However, it is possible that, with more discoveries, the distinct species types will hold.
Australopithecus bahrelghazali is dated to within the time period of Au. afarensis (3.6 mya; Brunet et al. 1995) and was the first Australopithecine to be discovered in Chad in central Africa. Researchers argue that the holotype, whom discoverers have named “Abel,” falls under the range of variation of Au. afarensis and therefore that A. bahrelghazali does not fall into a new species (Lebatard et al. 2008). If “Abel” is a member of Au. afarensis, the geographic range of the species would be greatly extended.
On a different note, Australopithecus deyiremada (meaning “close relative” in the Ethiopian language of Afar) is dated to 3.5 mya to 3.3 mya and is based on fossil mandible bones discovered in 2011 in Woranso-Mille (in the Afar region of Ethiopia) by Yohannes Haile-Selassie, an Ethiopian paleoanthropologist (Haile-Selassie et al. 2019). The discovery indicated, in contrast to Au. afarensis, smaller teeth with thicker enamel (potentially suggesting a harder diet) as well as a larger mandible and more projecting cheekbones. This find may be evidence that more than one closely related hominin species occupied the same region at the same temporal period (Haile-Selassie et al. 2015; Spoor 2015) or that other Au. afarensis specimens have been incorrectly designated. However, others have argued that this species has been prematurely identified and that more evidence is needed before splitting the taxa, since the variation appears subtle and may be due to slightly different niche occupations between populations over time.
Australopithecus garhi is another species found in the Middle Awash region of Ethiopia. It is currently dated to 2.5 mya (younger than Au. afarensis). Researchers have suggested it fills in a much-needed temporal “gap” between hominin finds in the region, with some anatomical differences, such as a relatively large cranial capacity (450 cc) and larger hind dentition than seen in other gracile Australopithecines. Similarly, the species has been argued to have longer hind limbs than Au. afarensis, although it was still able to move arboreally (Asfaw et al. 1999). However, this species is not well documented or understood and is based on only several fossil specimens. More astonishingly, crude stone tools resembling Oldowan (which will be described later) have been found in association with Au. garhi. While lacking some of the features of the Oldowan, this is one of the earliest technologies found in direct association with a hominin.
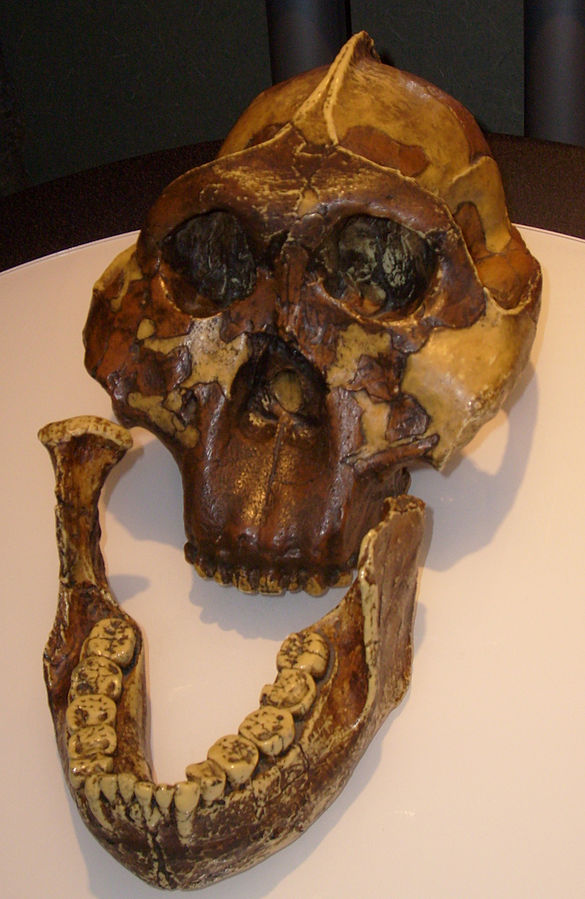
Kenyanthopus platyops (the name “platyops” refers to its flatter-faced appearance) is a highly contested genus/species designation of a specimen (KNM-WT 40000) from Lake Turkana in Kenya, discovered by Maeve Leakey in 1999 (Figure 9.11). Dated to between 3.5 mya and 3.2 mya, some have suggested this specimen is an Australopithecus, perhaps even Au. afarensis (with a brain size which is difficult to determine, yet appears small), while still others have placed this specimen in Homo (small dentition and flat-orthognathic face). While taxonomic placing of this species is quite divided, the discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis (Leakey et al. 2001). Some researchers have additionally associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this specimen.

The Genus Australopithecus
The Australopithecines are a diverse group of hominins, comprising various species. Australopithecus is the given group or genus name. It stems from the Latin word Australo, meaning “southern,” and the Greek word pithecus, meaning “ape.” Within this section, we will outline these differing species’ geological and temporal distributions across Africa, unique derived and/or shared traits, and importance in the fossil record.
Between 3 mya and 1 mya, there seems to be differences in dietary strategy between different species of hominins designated as Australopithecines. A pattern of larger posterior dentition (even relative to the incisors and canines in the front of the mouth), thick enamel, and cranial evidence for extremely large chewing muscles is far more pronounced in a group known as the robust australopithecines. This pattern is extreme relative to their earlier contemporaries or predecessors, the gracile australopithecines, and is certainly larger than those seen in early Homo, which emerged during this time. This pattern of incredibly large hind dentition (and very small anterior dentition) has led people to refer to robust australopithecines as megadont hominins (Figure 9.12).
Because of these differences, this section has been divided into “gracile” and “robust” Australopithecines, highlighting the morphological differences between the two groups (which many researchers have designated as separate genera: Australopithecus and Paranthropus, respectively) and then focusing on the individual species. It is worth noting, however, that not all researchers accept these clades as biologically or genetically distinct, with some researchers insisting that the relative gracile and robust features found in these species are due to parallel evolutionary events toward similar dietary niches.
Despite this genus’ ancestral traits and small cranial capacity, all members show evidence of bipedal locomotion. It is generally accepted that Australopithecus species display varying degrees of arborealism along with bipedality.
Gracile Australopithecines
This section describes individual species from across Africa. These species are called “gracile australopithecines” because of their smaller and less robust features compared to the divergent “robust” group. Numerous Australopithecine species have been named, but some are only based on a handful of fossil finds, whose designations are controversial.
East African Australopithecines
East African Australopithecines are found throughout the EARS, and they include the earliest species associated with this genus. Numerous fossil-yielding sites, such as Olduvai, Turkana, and Laetoli, have excellent, datable stratigraphy, owing to the layers of volcanic tufts that have accumulated over millions of years. These tufts may be dated using absolute dating techniques, such as Potassium-Argon dating (described in Chapter 7). This means that it is possible to know a relatively refined date for any fossil if the context (i.e., exact location) of that find is known. Similarly, comparisons between the faunal assemblages of these stratigraphic layers have allowed researchers to chronologically identify environmental changes.
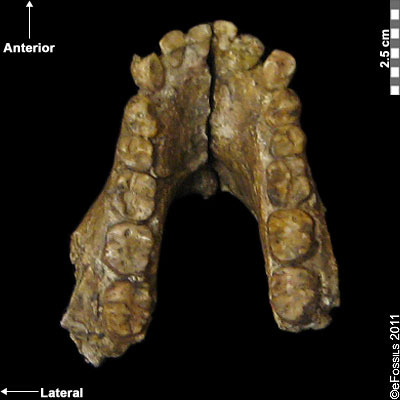
The earliest known Australopithecine is dated to 4.2 mya to 3.8 mya. Australopithecus anamensis (after “Anam,” meaning “lake” from the Turkana region in Kenya; Leakey et al. 1995; Patterson and Howells 1967) is currently found from sites in the Turkana region (Kenya) and Middle Awash (Ethiopia; Figure 9.13). Recently, a 2019 find from Ethiopia, named MRD, after Miro Dora where it was found, was discovered by an Ethiopian herder named Ali Bereino. It is one of the most complete cranial finds of this species (Ward et al. 1999). A small brain size (370 cc), relatively large canines, projecting cheekbones, and earholes show more ancestral features as compared to those of more recent Australopithecines. The most important element discovered with this species is a fragment of a tibia (shinbone), which demonstrates features associated with weight transfer during bipedal walking. Similarly, the earliest found hominin femur belongs to this species. Ancestral traits in the upper limb (such as the humerus) indicate some retained arboreal locomotion.
Some researchers suggest that Au. anamensis is an intermediate form of the chronospecies that becomes Au. afarensis, evolving from Ar. ramidus. However, this is debated, with other researchers suggesting morphological similarities and affinities with more recent species instead. Almost 100 specimens, representing over 20 individuals, have been found to date (Leakey et al. 1995; McHenry 2009; Ward et al. 1999).
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. Au. afarensis (which means “from the Afar region”) is dated to between 2.9 mya and 3.9 mya and is found in sites all along the EARS system, in Tanzania, Kenya, and Ethiopia (Figure 9.14). The most famous individual from this species is a partial female skeleton discovered in Hadar (Ethiopia), later nicknamed “Lucy,” after the Beatles’ song “Lucy in the Sky with Diamonds,” which was played in celebration of the find (Johanson et al. 1978; Kimbel and Delezene 2009). This skeleton was found in 1974 by Donald Johanson and dates to approximately 3.2 mya. In addition, in 2002 a juvenile of the species was found by Zeresenay Alemseged and given the name “Selam” (meaning “peace,” DIK 1-1), though it is popularly known as “Lucy’s Child” or as the “Dikika Child” (Alemseged et al. 2006). Similarly, the “Laetoli Footprints” (discussed in Chapter 7; Hay and Leakey 1982; Leakey and Hay 1979) have drawn much attention.

The canines and molars of Au. afarensis are reduced relative to great apes but are larger than those found in modern humans (indicative of a generalist diet); in addition, Au. afarensis has a prognathic face (the face below the eyes juts anteriorly) and robust facial features that indicate relatively strong chewing musculature (compared with Homo) but which are less extreme than in Paranthropus. Despite a reduction in canine size in this species, large overall size variation indicates high levels of sexual dimorphism.
Skeletal evidence indicates that this species was bipedal, as its pelvis and lower limb demonstrate a humanlike femoral neck, valgus knee, and bowl-shaped hip (Figure 9.15). More evidence of bipedalism is found in the footprints of this species. Au. afarensis is associated with the Laetoli Footprints, a 24-meter trackway of hominin fossil footprints preserved in volcanic ash discovered by Mary Leakey in Tanzania and dated to 3.5 mya to 3 mya. This set of prints is thought to have been produced by three bipedal individuals as there are no knuckle imprints, no opposable big toes, and a clear arch is present. The infants of this species are thought to have been more arboreal than the adults, as discovered through analyses of the foot bones of the Dikika Child dated to 3.32 mya (Alemseged et al. 2006).
Although not found in direct association with stone tools, potential evidence for cut marks on bones, found at Dikika, and dated to 3.39 mya indicates a possible temporal/ geographic overlap between meat eating, tool use, and this species. However, this evidence is fiercely debated. Others have associated the cut marks with the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species.
South African Australopithecines
Since the discovery of the Taung Child, there have been numerous Australopithecine discoveries from the region known as “The Cradle of Humankind,” which was recently given UNESCO World Heritage Site status as “The Fossil Hominid Sites of South Africa.” The limestone caves found in the Cradle allow for the excellent preservation of fossils. Past animals navigating the landscape and falling into cave openings, or caves used as dens by carnivores, led to the accumulation of deposits over millions of years. Many of the hominin fossils, encased in breccia (hard, calcareous sedimentary rock), are recently exposed from limestone quarries mined in the previous century. This means that extracting fossils requires excellent and detailed exposed work, often by a team of skilled technicians.
While these sites have historically been difficult to date, with mixed assemblages accumulated over large time periods, advances in techniques such as uranium-series dating have allowed for greater accuracy. Historically, the excellent faunal record from East Africa has been used to compare sites based on relative dating, whereby environmental and faunal changes and extinction events allow us to know which hominin finds are relatively younger or older than others.
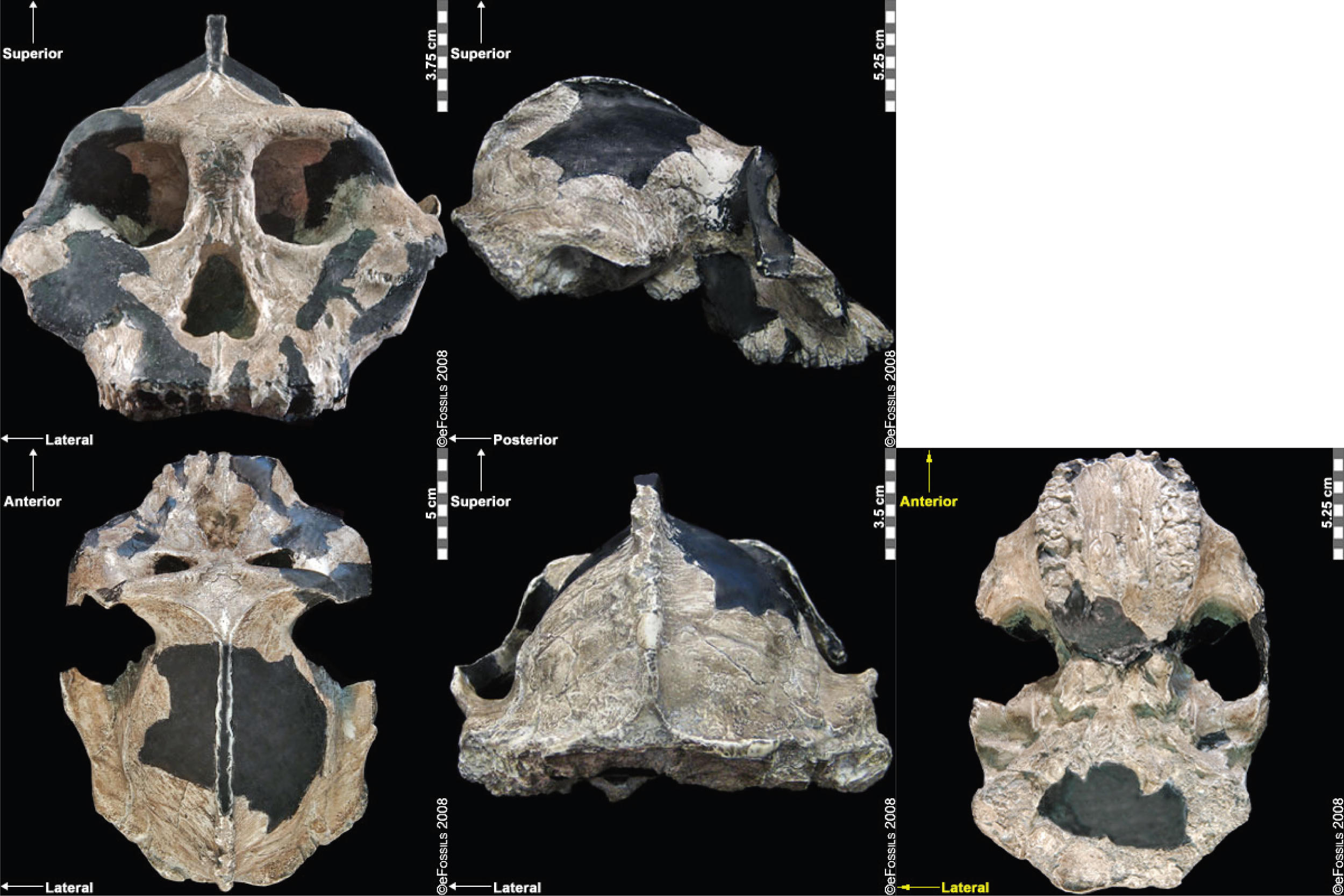
The discovery of the Taung Child in 1924 (discussed in the Special Topic box “The Taung Child” below) shifted the focus of palaeoanthropological research from Europe to Africa, although acceptance of this shift was slow (Broom 1947; Dart 1925). The species to which it is assigned, Australopithecus africanus (name meaning “Southern Ape of Africa”), is currently dated to between 3.3 mya and 2.1 mya (Pickering and Kramers 2010), with discoveries from Sterkfontein, Taung, Makapansgat, and Gladysvale in South Africa (Figure 9.16). A relatively large brain (400 cc to 500 cc), small canines without an associated diastema, and more rounded cranium and smaller teeth than Au. afarensis indicate some derived traits. Similarly, the postcranial remains (in particular, the pelvis) indicate bipedalism. However, the sloping face and curved phalanges (indicative of retained arboreal locomotor abilities) show some ancestral features. Although not in direct association with stone tools, a 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities.

Another famous Au. africanus skull (the skull of “Mrs. Ples”) was previously attributed to Plesianthropus transvaalensis, meaning “near human from the Transvaal,” the old name for Gauteng Province, South Africa (Broom 1947, 1950). The name was shortened by contemporary journalists to “Ples” (Figure 9.17). Due to the prevailing mores of the time, the assumed female found herself married, at least in name, and has become widely known as “Mrs. Ples.” It was later reassigned to Au. africanus and is now argued by some to be a young male rather than an adult female cranium (Thackeray 2000, Thackeray et al. 2002).
In 2008, nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger, noted a clavicle bone in some leftover mining breccia in the Malapa Fossil Site (South Africa). After rigorous studies, the species, Australopithecus sediba (meaning “fountain” or “wellspring” in the South African language of Sesotho), was named in 2010 (Figure 9.18; Berger et al. 2010). The first type specimen belongs to a juvenile male, Karabo (MH1), but the species is known from at least six partial skeletons, from infants through adults. These specimens are currently dated to 1.97 mya (Dirks et al. 2010). The discoverers have argued that Au. sediba shows mosaic features between Au. africanus and the genus, Homo, which potentially indicates a transitional species, although this is heavily debated. These features include a small brain size (Australopithecus-like; 420 cc to 450 cc) but gracile mandible and small teeth (Homo-like). Similarly, the postcranial skeletons are also said to have mosaic features: scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. Some researchers have argued that Au. sediba shows a modern hand morphology (shorter fingers and a longer thumb), indicating that adaptations to tool manufacture and use may be present in this species.
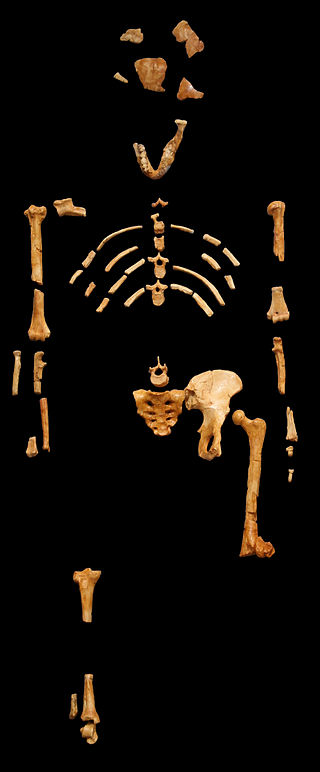
Another famous Australopithecine find from South Africa is that of the nearly complete skeleton now known as “Little Foot” (Clarke 1998, 2013). Little Foot (StW 573) is potentially the earliest dated South African hominin fossil, dating to 3.7 mya, based on radiostopic techniques, although some argue that it is younger than 3 mya (Pickering and Kramers 2010). The name is jokingly in contrast to the cryptid species “bigfoot” and is named because the initial discovery of four ankle bones indicated bipedality. Little Foot was discovered by Ron Clarke in 1994, when he came across the ankle bones while sorting through monkey fossils in the University of Witwatersrand collections (Clarke and Tobias 1995). He asked Stephen Motsumi and Nkwane Molefe to identify the known records of the fossils, which allowed them to find the rest of the specimen within just days of searching the Sterkfontein Caves’ Silberberg Grotto.
The discoverers of Little Foot insist that other fossil finds, previously identified as Au. Africanus, be placed in this new species based on shared ancestral traits with older East African Australopithecines (Clarke and Kuman 2019). These include features such as a relatively large brain size (408 cc), robust zygomatic arch, and a flatter midface. Furthermore, the discoverers have argued that the heavy anterior dental wear patterns, relatively large anterior dentition, and smaller hind dentition of this specimen more closely resemble that of Au. anamensis or Au. afarensis. It has thus been placed in the species Australopithecus prometheus. This species name refers to a previously defunct taxon named by Raymond Dart. The species designation was, through analyzing Little Foot, revived by Ron Clarke, who insists that many other fossil hominin specimens have prematurely been placed into Au. africanus. Others say that it is more likely that Au. africanus is a more variable species and not representative of two distinct species.
Paranthropus “Robust” Australopithecines
In the robust australopithecines, the specialized nature of the teeth and masticatory system, such as flaring zygomatic arches (cheekbones), accommodate very large temporalis (chewing) muscles.These features also include a large, broad, dish-shaped face and and a large mandible with extremely large posterior dentition (referred to as megadonts) and hyper-thick enamel (Kimbel 2015; Lee-Thorp 2011; Wood 2010). Research has revolved around the shared adaptations of these “robust” australopithecines, linking their morphologies to a diet of hard and/or tough foods (Brain 1967; Rak 1988). Some argued that the diet of the robust australopithecines was so specific that any change in environment would have accelerated their extinction. The generalist nature of the teeth of the gracile australopithecines, and of early Homo, would have made them more capable of adapting to environmental change. However, some have suggested that the features of the robust australopithecines might have developed as an effective response to what are known as fallback foods in hard times rather than indicating a lack of adaptability.
There are currently three widely accepted robust australopithecus or, Paranthropus, species: P. aethiopicus, which has more ancestral traits, and P. boisei and P. robustus, which are more derived in their features (Strait et al. 1997; Wood and Schroer 2017). These three species have been grouped together by a majority of scholars as a single genus as they share more derived features (are more closely related to each other; or, in other words, are monophyletic) than the other australopithecines (Grine 1988; Hlazo 2015; Strait et al. 1997; Wood 2010 ). While researchers have mostly agreed to use the umbrella term Paranthropus, there are those who disagree (Constantino and Wood 2004, 2007; Wood 2010).
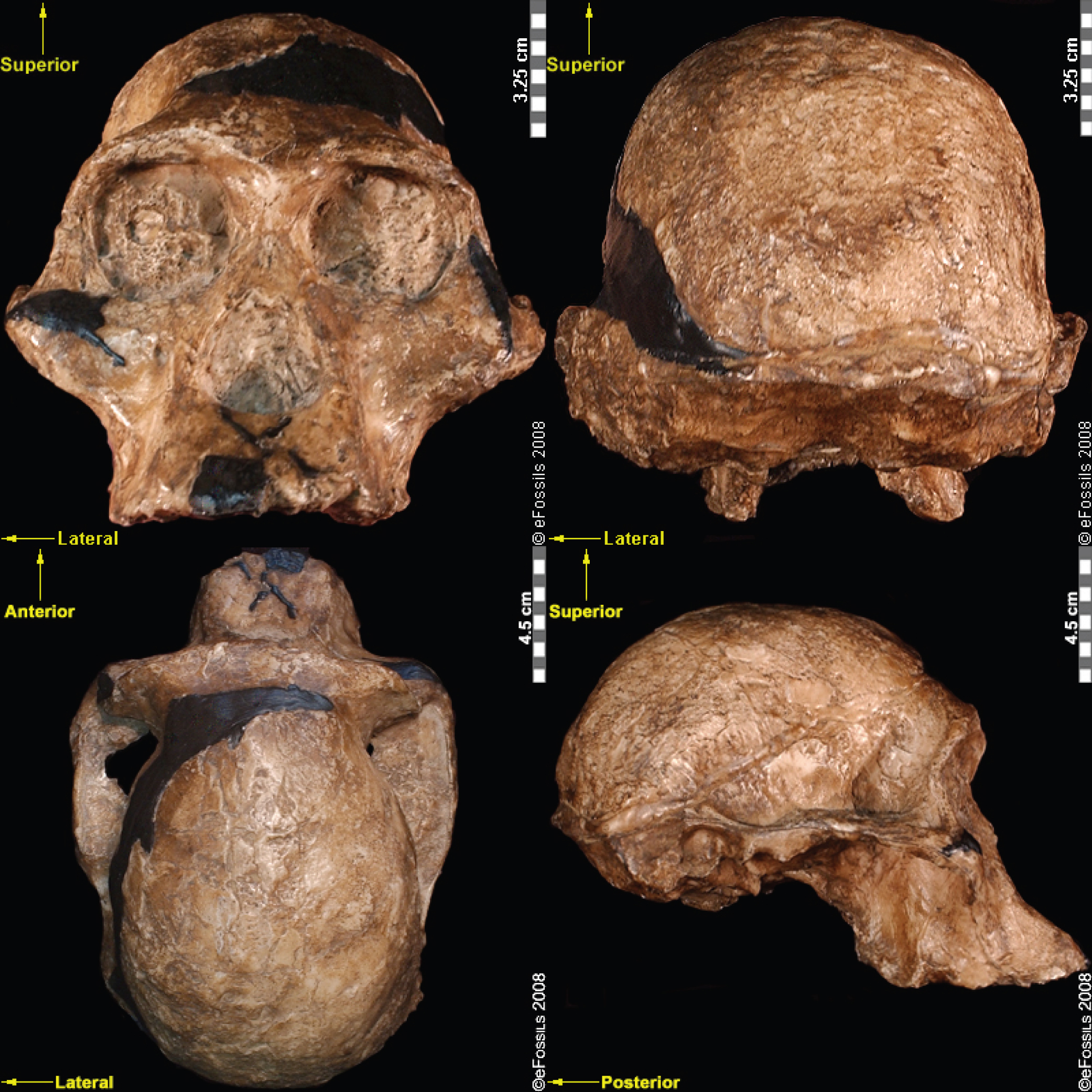
As a collective, this genus spans 2.7 mya to 1.0 mya, although the dates of the individual species differ. The earliest of the Paranthropus species, Paranthropus aethiopicus, is dated to between 2.7 mya and 2.3 mya and currently found in Tanzania, Kenya, and Ethiopia in the EARS system (Figure 9.19; Constantino and Wood 2007; Hlazo 2015; Kimbel 2015; Walker et al. 1986; White 1988). It is well known because of one specimen known as the “Black Skull” (KNM–WT 17000), so called because of the mineral manganese that stained it black during fossilization (Kimbel 2015). As with all robust Australopithecines, P. aethiopicus has the shared derived traits of large, flat premolars and molars; large, flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle); a sagittal crest (ridge on the top of the skull) for increased muscle attachment of the chewing muscles to the skull; and a robust mandible and supraorbital torus (brow ridge). However, only a few teeth have been found. A proximal tibia indicates bipedality and similar body size to Au. afarensis. In recent years, researchers have discovered and assigned a proximal tibia and juvenile cranium (L.338y-6) to the species (Wood and Boyle 2016).
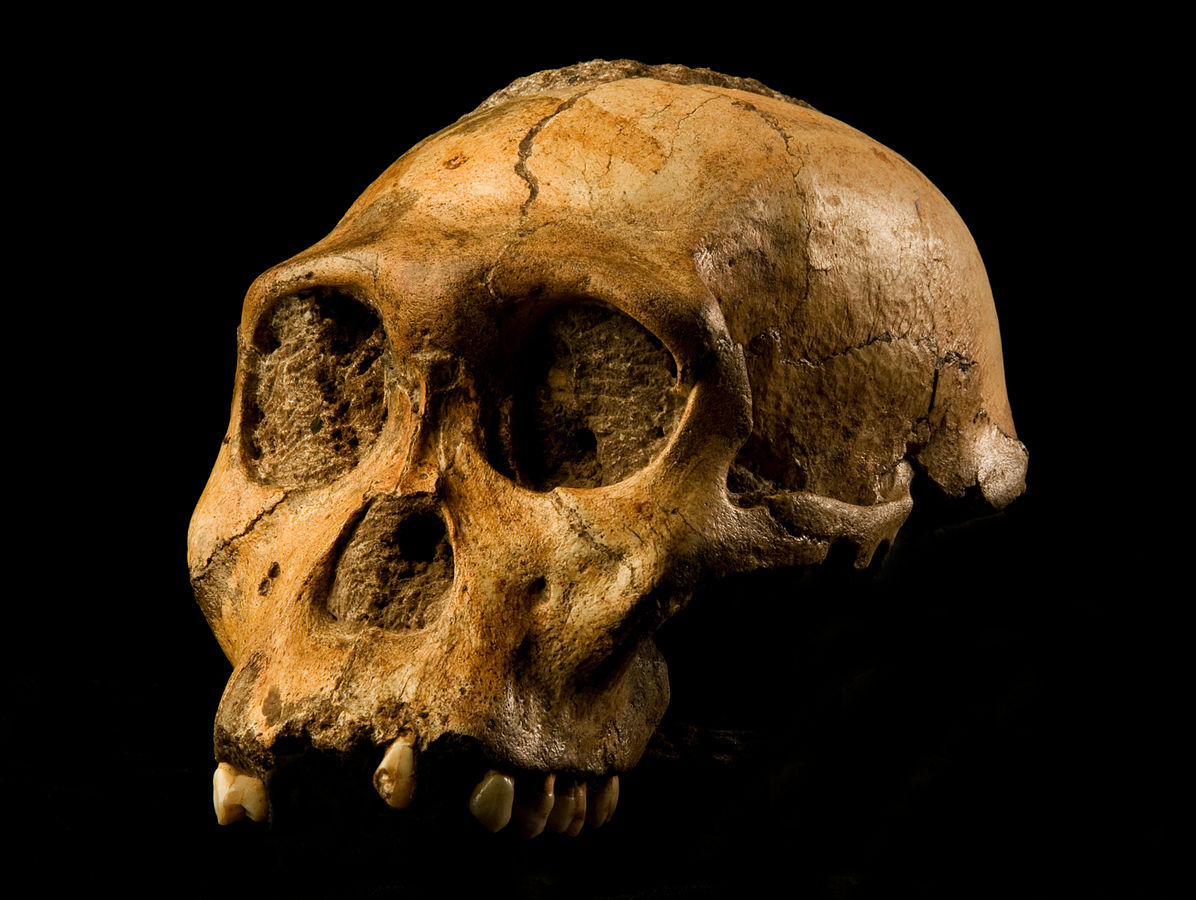
First attributed as Zinjanthropus boisei (with the first discovery going by the nickname “Zinj” or sometimes “Nutcracker Man”), Paranthropus boisei was discovered in 1959 by Mary Leakey (see Figure 9.20 and 9.21; Hay 1990; Leakey 1959). This “robust” australopith species is distributed across countries in East Africa at sites such as Kenya (Koobi Fora, West Turkana, and Chesowanja), Malawi (Malema-Chiwondo), Tanzania (Olduvai Gorge and Peninj), and Ethiopia (Omo River Basin and Konso). The hypodigm, sample of fossils whose features define the group, has been found by researchers to date to roughly 2.4 mya to 1.4 mya. Due to the nature of its exaggerated, larger, and more robust features, P. boisei has been termed hyper-robust—that is, even more heavily built than other robust species, with very large, flat posterior dentition (Kimbel 2015). Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). Another famous specimen from this species is the Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu.

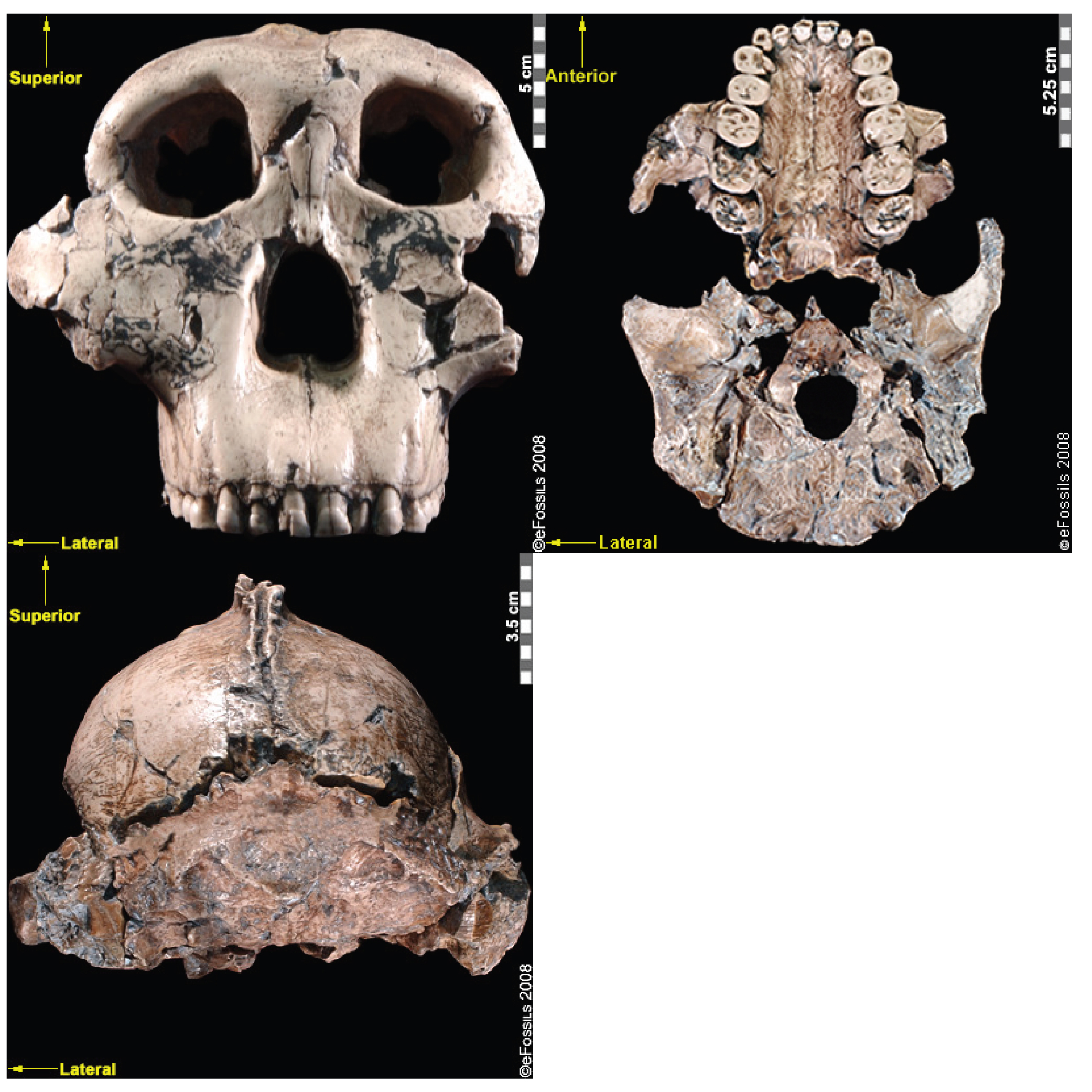
Paranthropus robustus was the first taxon to be discovered within the genus in Kromdraai B by a schoolboy named Gert Terblanche; subsequent fossil discoveries were made by researcher Robert Broom in 1938 (Figure 9.22; Broom 1938a, 1938b, 1950), with the holotype specimen TM 1517 (Broom 1938a, 1938b, 1950; Hlazo 2018). Paranthropus robustus dates approximately from 2.0 mya to 1 mya and is the only taxon from the genus to be discovered in South Africa. Several of these fossils are fragmentary in nature, distorted, and not well preserved because they have been recovered from quarry breccia using explosives. P. robustus features are neither as “hyper-robust” as P. boisei nor as ancestral as P. aethiopicus; instead, they have been described as being less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring; Rak 1983; Walker and Leakey 1988). Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick-enameled dentition.
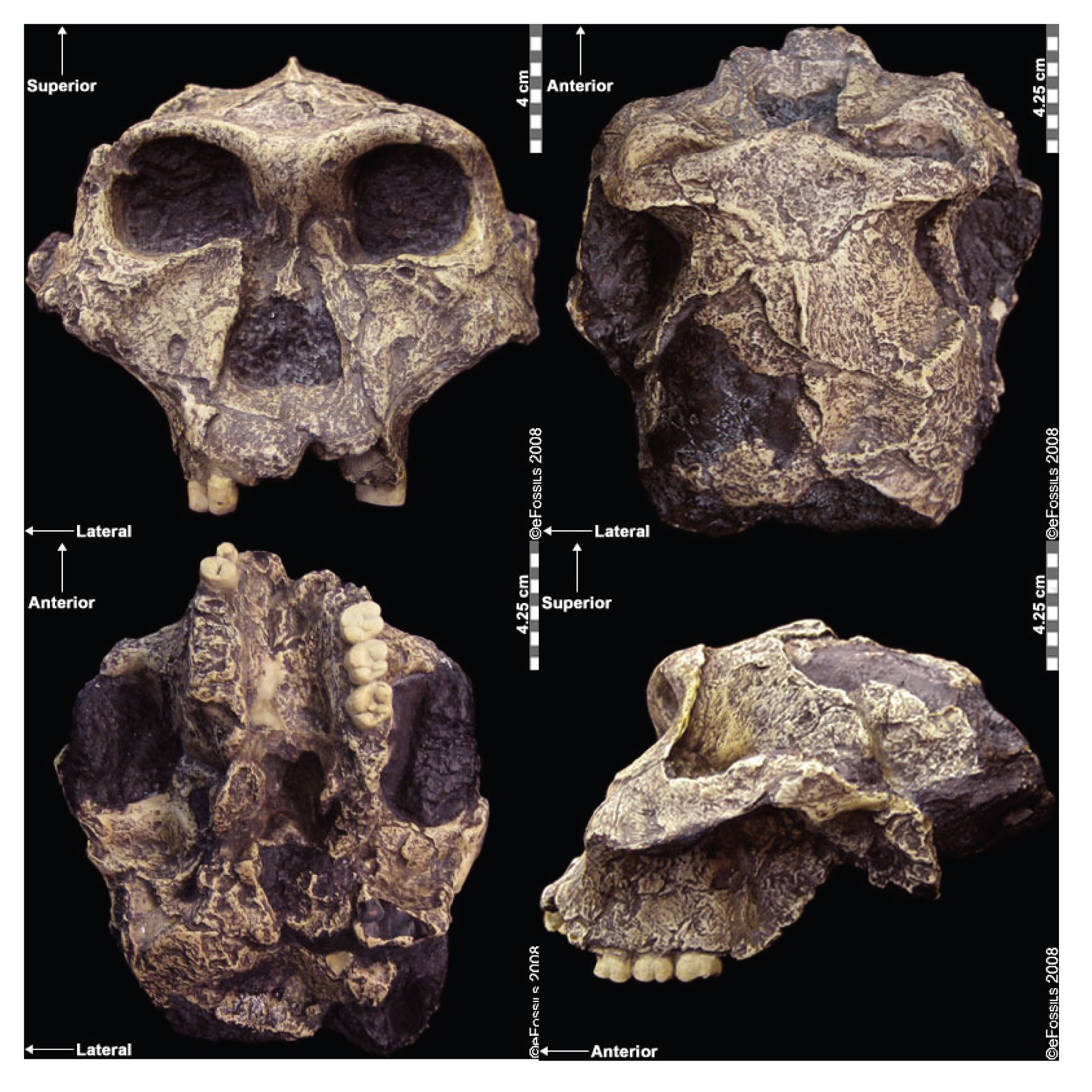
Comparisons between Gracile and Robust Australopiths
Comparisons between gracile and robust australopithecines may indicate different phylogenetic groupings or parallel evolution in several species. In general, the robust australopithecines have large temporalis (chewing) muscles, as indicated by flaring zygomatic arches, sagittal crests, and robust mandibles (jawbones). Their hind dentition is large (megadont), with low cusps and thick enamel. Within the gracile australopithecines, researchers have debated the relatedness of the species, or even whether these species should be lumped together to represent more variable or polytypic species. Often researchers will attempt to draw chronospecific trajectories, with one taxon said to evolve into another over time.
Special Topic: The Taung Child
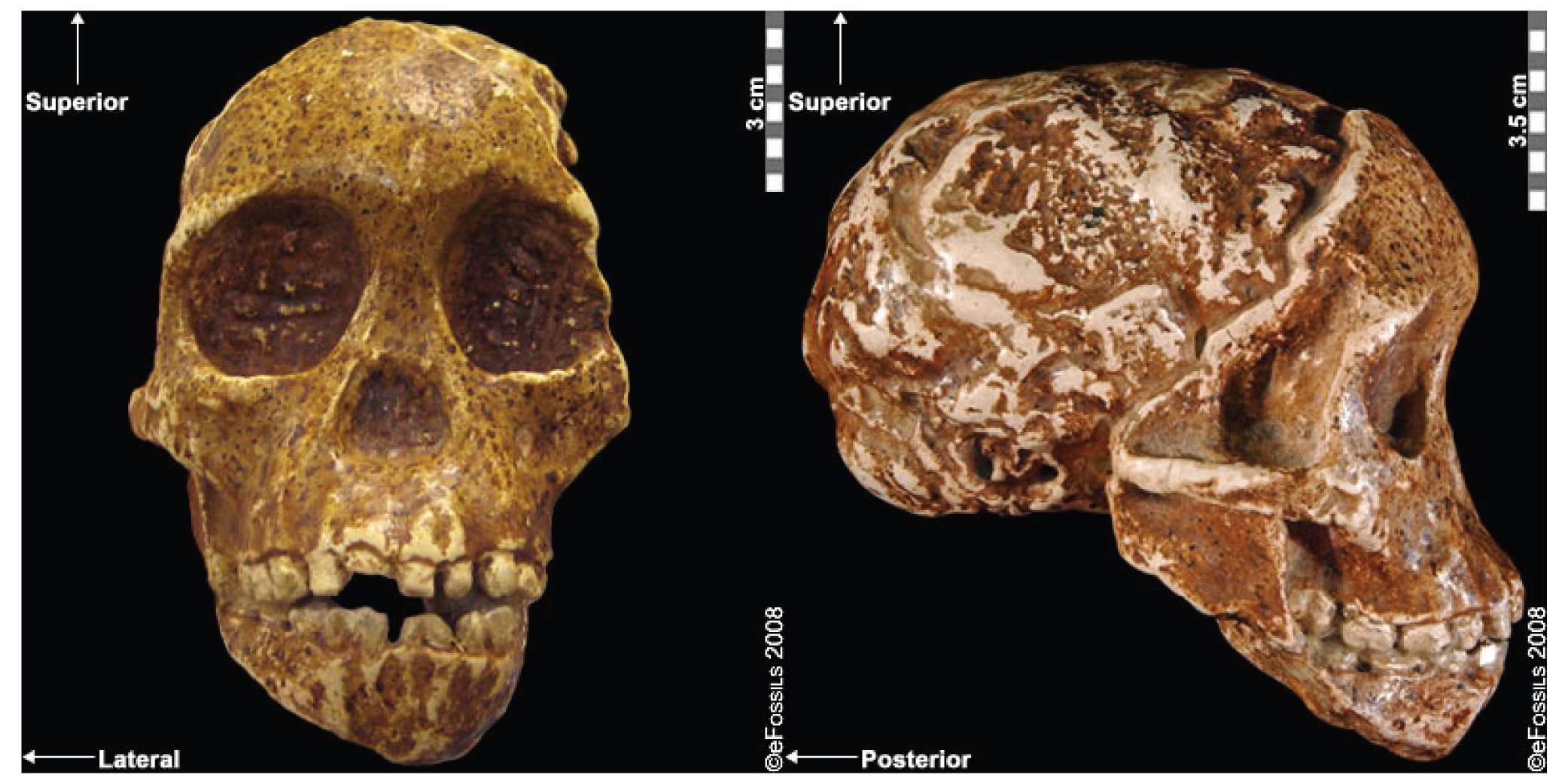
The well-known fossil of a juvenile Australopithecine, the “Taung Child,” was the first early hominin evidence ever discovered and was the first to demonstrate our common human heritage in Africa (Figure 9.23; Dart 1925). The tiny facial skeleton and natural endocast were discovered in 1924 by a local quarryman in the North West Province in South Africa and were painstakingly removed from the surrounding cement-like breccia by Raymond Dart using his wife’s knitting needles. When first shared with the scientific community in 1925, it was discounted as being nothing more than a young monkey of some kind. Prevailing biases of the time made it too difficult to contemplate that this small-brained hominin could have anything to do with our own history. The fact that it was discovered in Africa simply served to strengthen this bias.
Early Tool Use and Technology
Early Stone Age Technology (ESA)
The Early Stone Age (ESA) marks the beginning of recognizable technology made by our human ancestors. Stone-tool (or lithic) technology is defined by the fracturing of rocks and the manufacture of tools through a process called knapping. The Stone Age lasted for more than 3 million years and is broken up into chronological periods called the Early (ESA), Middle (MSA), and Later Stone Ages (LSA). Each period is further broken up into a different techno-complex, a term encompassing multiple assemblages (collections of artifacts) that share similar traits in terms of artifact production and morphology. The ESA spanned the largest technological time period of human innovation from over 3 million years ago to around 300,000 years ago and is associated almost entirely with hominin species prior to modern Homo sapiens. As the ESA advanced, stone tool makers (known as knappers) began to change the ways they detached flakes and eventually were able to shape artifacts into functional tools. These advances in technology go together with the developments in human evolution and cognition, dispersal of populations across the African continent and the world, and climatic changes.
In order to understand the ESA, it is important to consider that not all assemblages are exactly the same within each techno-complex: one can have multiple phases and traditions at different sites (Lombard et al. 2012). However, there is an overarching commonality between them. Within stone tool assemblages, both flakes or cores (the rocks from which flakes are removed) are used as tools. Large Cutting Tools (LCTs) are tools that are shaped to have functional edges. It is important to note that the information presented here is a small fraction of what is known about the ESA, and there are ongoing debates and discoveries within archaeology.
Currently, the oldest-known stone tools, which form the techno-complex the Lomekwian, date to 3.3 mya (Harmand et al. 2015; Toth 1985). They were found at a site called Lomekwi 3 in Kenya. This techno-complex is the most recently defined and pushed back the oldest-known date for lithic technology. There is only one known site thus far and, due to the age of the site, it is associated with species prior to Homo, such as Kenyanthropus platyops. Flakes were produced through indirect percussion, whereby the knappers held a rock and hit it against another rock resting on the ground. The pieces are very chunky and do not display the same fracture patterns seen in later techno-complexes. Lomekwian knappers likely aimed to get a sharp-edged piece on a flake, which would have been functional, although the specific function is currently unknown.
Stone tool use, however, is not only understood through the direct discovery of the tools. Cut marks on fossilized animal bones may illuminate the functionality of stone tools. In one controversial study in 2010, researchers argued that cut marks on a pair of animal bones from Dikika (Ethiopia), dated to 3.4 mya, were from stone tools. The discoverers suggested that they be more securely associated, temporally, with Au. afarensis. However, others have noted that these marks are consistent with teeth marks from crocodiles and other carnivores.
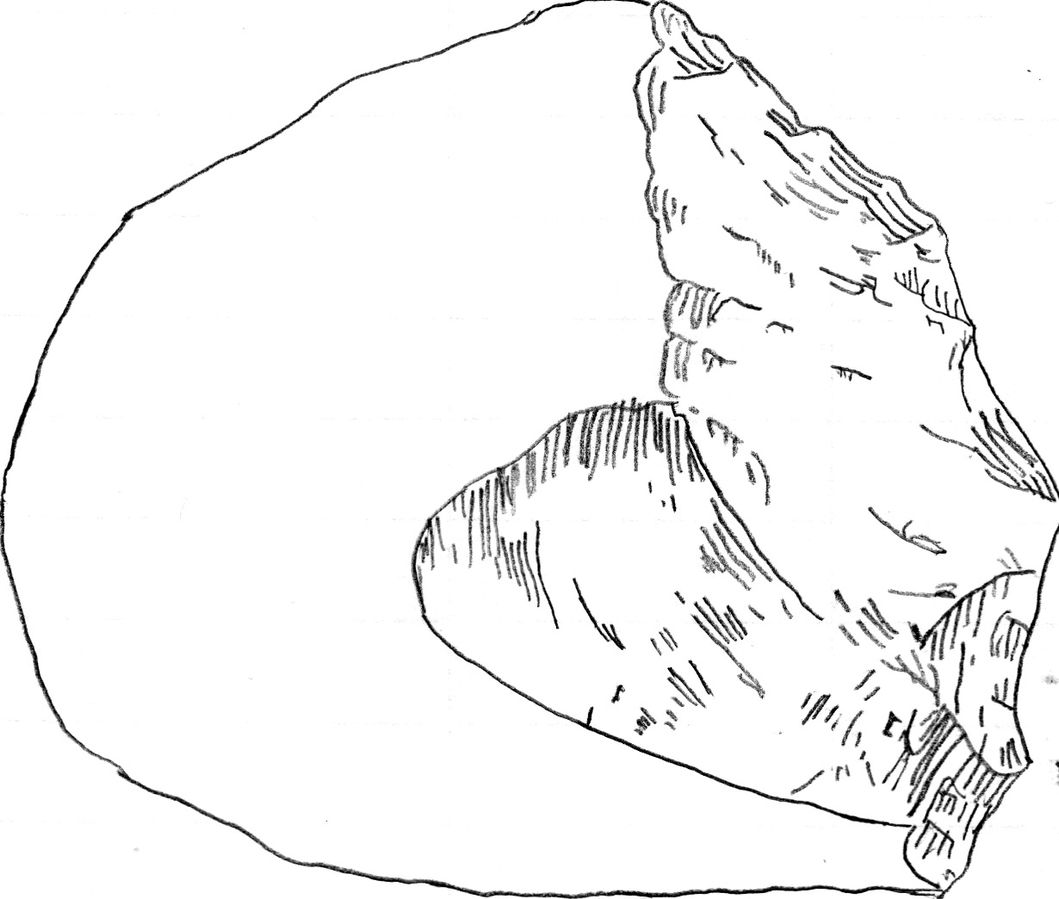
The Oldowan techno-complex is far more established in the scientific literature (Leakey 1971). It is called the Oldowan because it was originally discovered in Olduvai Gorge, Tanzania, but the oldest assemblage is from Gona in Ethiopia, dated to 2.6 mya (Semaw 2000). The techno-complex is defined as a core and flake industry. Like the Lomekwian, there was an aim to get sharp-edged flakes, but this was achieved through a different production method. Knappers were able to actively hold or manipulate the core being knapped, which they could directly hit using a hammerstone. This technique is known as free-hand percussion, and it demonstrates an understanding of fracture mechanics. It has long been argued that the Oldowan hominins were skillful in tool manufacture.
Because Oldowan knapping requires skill, earlier researchers have attributed these tools to members of our genus, Homo. However, some have argued that these tools are in more direct association with hominins in the genera described in this chapter (Figure 9.24).
Invisible Tool Manufacture and Use
The vast majority of our understanding of these early hominins comes from fossils and reconstructed paleoenvironments. It is only from 3 mya when we can start “looking into their minds” and lifestyles by analyzing their manufacture and use of stone tools. However, the vast majority of tool use in primates (and, one can argue, in humans) is not with durable materials like stone. All of our extant great ape relatives have been observed using sticks, leaves, and other materials for some secondary purpose (to wade across rivers, to “fish” for termites, or to absorb water for drinking). It is possible that the majority of early hominin tool use and manufacture may be invisible to us because of this preservation bias.
Chapter Summary
The fossil record of our earliest hominin relatives has allowed paleoanthropologists to unpack some of the mysteries of our evolution. We now know that traits associated with bipedalism evolved before other “human-like” traits, even though the first hominins were still very capable of arboreal locomotion. We also know that, for much of this time, hominin taxa were diverse in the way they looked and what they ate, and they were widely distributed across the African continent. And we know that the environments in which these hominins lived underwent many changes over this time during several warming and cooling phases.
Yet this knowledge has opened up many new mysteries. We still need to better differentiate some taxa. In addition, there are ongoing debates about why certain traits evolved and what they meant for the extinction of some of our relatives (like the robust australopiths). The capabilities of these early hominins with respect to tool use and manufacture is also still uncertain.
Hominin Species Summaries
|
Hominin |
Sahelanthropus tchadensis |
|---|---|
|
Dates |
7 mya to 6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
The initial discovery, made in 2001. |
|
Brain size |
360 cc average |
|
Dentition |
Smaller than in extant great apes; larger and pointier than in humans. Canines worn at the tips. |
|
Cranial features |
A short cranial base and a foramen magnum (hole in which the spinal cord enters the cranium) that is more humanlike in positioning; has been argued to indicate upright walking. |
|
Postcranial features |
Currently little published postcranial material. |
|
Culture |
N/A |
|
Other |
The extent to which this hominin was bipedal is currently heavily debated. If so, it would indicate an arboreal bipedal ancestor of hominins, not a knuckle-walker like chimpanzees. |
|
Hominin |
Orrorin tugenensis |
|---|---|
|
Dates |
6 mya to 5.7 mya |
|
Region(s) |
Tugen Hills (Kenya) |
|
Famous discoveries |
Original discovery in 2000. |
|
Brain size |
N/A |
|
Dentition |
Smaller cheek teeth (molars and premolars) than even more recent hominins (i.e., derived), thick enamel, and reduced, but apelike, canines. |
|
Cranial features |
Not many found |
|
Postcranial features |
Fragmentary leg, arm, and finger bones have been found. Indicates bipedal locomotion. |
|
Culture |
Potential toolmaking capability based on hand morphology, but nothing found directly. |
|
Other |
This is the earliest species that clearly indicates adaptations for bipedal locomotion. |
|
Hominin |
Ardipithecus kadabba |
|---|---|
|
Dates |
5.2 mya to 5.8 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
Discovered by Yohannes Haile-Selassie in 1997. |
|
Brain size |
N/A |
|
Dentition |
Larger hind dentition than in modern chimpanzees. Thick enamel and larger canines than in later hominins. |
|
Cranial features |
N/A |
|
Postcranial features |
A large hallux (big toe) bone indicates a bipedal “push off.” |
|
Culture |
N/A |
|
Other |
Faunal evidence indicates a mixed grassland/woodland environment. |
|
Hominin |
Ardipithecus ramidus |
|---|---|
|
Dates |
4.4 mya |
|
Region(s) |
Middle Awash region and Gona (Ethiopia) |
|
Famous discoveries |
A partial female skeleton nicknamed “Ardi” (ARA-VP-6/500) (found in 1994). |
|
Brain size |
300 cc to 350 cc |
|
Dentition |
Little differences between the canines of males and females (small sexual dimorphism). |
|
Cranial features |
Midfacial projection, slightly prognathic. Cheekbones less flared and robust than in later hominins. |
|
Postcranial features |
Ardi demonstrates a mosaic of ancestral and derived characteristics in the postcrania. For instance, an opposable big toe similar to chimpanzees (i.e., more ancestral), which could have aided in climbing trees effectively. However, the pelvis and hip show that she could walk upright (i.e., it is derived), supporting her hominin status. |
|
Culture |
None directly associated |
|
Other |
Over 110 specimens from Aramis |
|
Hominin |
Australopithecus anamensis |
|---|---|
|
Dates |
4.2 mya to 3.8 mya |
|
Region(s) |
Turkana region (Kenya); Middle Awash (Ethiopia) |
|
Famous discoveries |
A 2019 find from Ethiopia, named MRD. |
|
Brain size |
370 cc |
|
Dentition |
Relatively large canines compared with more recent Australopithecines. |
|
Cranial features |
Projecting cheekbones and ancestral earholes. |
|
Postcranial features |
Lower limb bones (tibia and femur) indicate bipedality; arboreal features in upper limb bones (humerus) found. |
|
Culture |
N/A |
|
Other |
Almost 100 specimens, representing over 20 individuals, have been found to date. |
|
Hominin |
Australopithecus afarensis |
|---|---|
|
Dates |
3.9 mya to 2.9 mya |
|
Region(s) |
Afar Region, Omo, Maka, Fejej, and Belohdelie (Ethiopia); Laetoli (Tanzania); Koobi Fora (Kenya) |
|
Famous discoveries |
Lucy (discovery: 1974), Selam (Dikika Child, discovery: 2000), Laetoli Footprints (discovery: 1976). |
|
Brain size |
380 cc to 430 cc |
|
Dentition |
Reduced canines and molars relative to great apes but larger than in modern humans. |
|
Cranial features |
Prognathic face, facial features indicate relatively strong chewing musculature (compared with Homo) but less extreme than in Paranthropus. |
|
Postcranial features |
Clear evidence for bipedalism from lower limb postcranial bones. Laetoli Footprints indicate humanlike walking. Dikika Child bones indicate retained ancestral arboreal traits in the postcrania. |
|
Culture |
None directly, but close in age and proximity to controversial cut marks at Dikika and early tools in Lomekwi. |
|
Other |
Au. afarensis is one of the oldest and most well-known australopithecine species and consists of a large number of fossil remains. |
|
Hominin |
Australopithecus bahrelghazali |
|---|---|
|
Dates |
3.6 mya |
|
Region(s) |
Chad |
|
Famous discoveries |
“Abel,” the holotype (discovery: 1995). |
|
Brain size |
N/A |
|
Dentition |
N/A |
|
Cranial features |
N/A |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Arguably within range of variation of Au. afarensis. |
|
Hominin |
Australopithecus prometheus |
|---|---|
|
Dates |
3.7 mya (debated) |
|
Region(s) |
Sterkfontein (South Africa) |
|
Famous discoveries |
“Little Foot” (StW 573) (discovery: 1994) |
|
Brain size |
408 cc (Little Foot estimate) |
|
Dentition |
Heavy anterior dental wear patterns, relatively large anterior dentition and smaller hind dentition, similar to Au. afarensis. |
|
Cranial features |
Relatively larger brain size, robust zygomatic arch, and a flatter midface. |
|
Postcranial features |
The initial discovery of four ankle bones indicated bipedality. |
|
Culture |
N/A |
|
Other |
Highly debated new species designation. |
|
Hominin |
Australopithecus deyiremada |
|---|---|
|
Dates |
3.5 mya to 3.3 mya |
|
Region(s) |
Woranso-Mille (Afar region, Ethiopia) |
|
Famous discoveries |
First fossil mandible bones were discovered in 2011 in the Afar region of Ethiopia by Yohannes Haile-Selassie. |
|
Brain size |
N/A |
|
Dentition |
Smaller teeth with thicker enamel than seen in Au. afarensis, with a potentially hardier diet. |
|
Cranial features |
Larger mandible and more projecting cheekbones than in Au. afarensis. |
|
Postcranial features |
N/A |
|
Culture |
N/A |
|
Other |
Contested species designation; arguably a member of Au. afarensis. |
|
Hominin |
Kenyanthopus platyops |
|---|---|
|
Dates |
3.5 mya to 3.2 mya |
|
Region(s) |
Lake Turkana (Kenya) |
|
Famous discoveries |
KNM–WT 40000 (discovered 1999) |
|
Brain size |
Difficult to determine but appears within the range of Australopithecus afarensis. |
|
Dentition |
Small molars/dentition (Homo-like characteristic) |
|
Cranial features |
Flatter (i.e., orthognathic) face |
|
Postcranial features |
N/A |
|
Culture |
Some have associated the earliest tool finds from Lomekwi, Kenya, temporally (3.3 mya) and in close geographic proximity to this species/specimen. |
|
Other |
Taxonomic placing of this species is quite divided. The discoverers have argued that this species is ancestral to Homo, in particular to Homo ruldolfensis. |
|
Hominin |
Australopithecus africanus |
|---|---|
|
Dates |
3.3 mya to 2.1 mya |
|
Region(s) |
Sterkfontein, Taung, Makapansgat, Gladysvale (South Africa) |
|
Famous discoveries |
Taung Child (discovery in 1994), “Mrs. Ples” (discover in 1947), Little Foot (arguable; discovery in 1994). |
|
Brain size |
400 cc to 500 cc |
|
Dentition |
Smaller teeth (derived) relative to Au. afarensis. Small canines with no diastema. |
|
Cranial features |
A rounder skull compared with Au. afarensis in East Africa. A sloping face (ancestral). |
|
Postcranial features |
Similar postcranial evidence for bipedal locomotion (derived pelvis) with retained arboreal locomotion, e.g., curved phalanges (fingers), as seen in Au. afarensis. |
|
Culture |
None with direct evidence. |
|
Other |
A 2015 study noted that the trabecular bone morphology of the hand was consistent with forceful tool manufacture and use, suggesting potential early tool abilities. |
|
Hominin |
Australopithecus garhi |
|---|---|
|
Dates |
2.5 mya |
|
Region(s) |
Middle Awash (Ethiopia) |
|
Famous discoveries |
N/A |
|
Brain size |
450 cc |
|
Dentition |
Larger hind dentition than seen in other gracile Australopithecines. |
|
Cranial features |
N/A |
|
Postcranial features |
A femur of a fragmentary partial skeleton, argued to belong to Au. garhi, indicates this species may be longer-limbed than Au. afarensis, although still able to move arboreally. |
|
Culture |
Crude stone tools resembling Oldowan (described later) have been found in association with Au. garhi. |
|
Other |
This species is not well documented or understood and is based on only a few fossil specimens. |
|
Hominin |
Paranthropus aethiopicus |
|---|---|
|
Dates |
2.7 mya to 2.3 mya |
|
Region(s) |
West Turkana (Kenya); Laetoli (Tanzania); Omo River Basin (Ethiopia) |
|
Famous discoveries |
The “Black Skull” (KNM–WT 17000) (discovery 1985). |
|
Brain Size |
410 cc |
|
Dentition |
P. aethiopicus has the shared derived traits of large flat premolars and molars, although few teeth have been found. |
|
Cranial features |
Large flaring zygomatic arches for accommodating large chewing muscles (the temporalis muscle), a sagittal crest for increased muscle attachment of the chewing muscles to the skull, and a robust mandible and supraorbital torus (brow ridge). |
|
Postcranial features |
A proximal tibia indicates bipedality and similar size to Au. afarensis. |
|
Culture |
N/A |
|
Other |
The “Black Skull” is so called because of the mineral manganese that stained it black during fossilization. |
|
Hominin |
Paranthropus boisei |
|---|---|
|
Dates |
2.4 mya to 1.4 mya |
|
Region(s) |
Koobi Fora, West Turkana, and Chesowanja (Kenya); Malema-Chiwondo (Malawi), Olduvai Gorge and Peninj (Tanzania); and Omo River basin and Konso (Ethiopia) |
|
Famous discoveries |
“Zinj,” or sometimes “Nutcracker Man” (OH5), in 1959 by Mary Leakey. The Peninj mandible from Tanzania, found in 1964 by Kimoya Kimeu. |
|
Brain size |
500 cc to 550 cc |
|
Dentition |
Very large, flat posterior dentition (largest of all hominins currently known). Much smaller anterior dentition. Very thick dental enamel. |
|
Cranial features |
Indications of very large chewing muscles (e.g., flaring zygomatic arches and a large sagittal crest). |
|
Postcranial features |
Evidence for high variability and sexual dimorphism, with estimates of males at 1.37 meters tall and females at 1.24 meters. |
|
Culture |
Richard Leakey and Bernard Wood have both suggested that P. boisei could have made and used stone tools. Tools dated to 2.5 mya in Ethiopia have been argued to possibly belong to this species. |
|
Other |
Despite the cranial features of P. boisei indicating a tough diet of tubers, nuts, and seeds, isotopes indicate a diet high in C4 foods (e.g., grasses, such as sedges). This differs from what is seen in P. robustus. |
|
Hominin |
Australopithecus sediba |
|---|---|
|
Dates |
1.97 mya |
|
Region(s) |
Malapa Fossil Site (South Africa) |
|
Famous discoveries |
Karabo (MH1) (discovery in 2008) |
|
Brain size |
420 cc to 450 cc |
|
Dentition |
Small dentition with Australopithecine cusp-spacing. |
|
Cranial features |
Small brain size (Australopithecus-like) but gracile mandible (Homo-like). |
|
Postcranial features |
Scientists have interpreted this mixture of traits (such as a robust ankle but evidence for an arch in the foot) as a transitional phase between a body previously adapted to arborealism (tree climbing, particularly in evidence from the bones of the wrist) to one that adapted to bipedal ground walking. |
|
Culture |
None of direct association, but some have argued that a modern hand morphology (shorter fingers and a longer thumb) means that adaptations to tool manufacture and use may be present in this species. |
|
Other |
It was first discovered through a clavicle bone in 2008 by nine-year-old Matthew Berger, son of paleoanthropologist Lee Berger. |
|
Hominin |
Paranthropus robustus |
|---|---|
|
Dates |
2.3 mya to 1 mya |
|
Region(s) |
Kromdraai B, Swartkrans, Gondolin, Drimolen, and Coopers Cave (South Africa) |
|
Famous discoveries |
SK48 (original skull) |
|
Brain size |
410 cc to 530 cc |
|
Dentition |
Large posterior teeth with thick enamel, consistent with other Robust Australopithecines. Enamel hypoplasia is also common in this species, possibly because of instability in the development of large, thick enameled dentition. |
|
Cranial features |
P. robustus features are neither as “hyper-robust” as P. boisei or as ancestral in features as P. aethiopicus. They have been described as less derived, more general features that are shared with both East African species (e.g., the sagittal crest and zygomatic flaring). |
|
Postcranial features |
Reconstructions indicate sexual dimorphism. |
|
Culture |
N/A |
|
Other |
Several of these fossils are fragmentary in nature, distorted, and not well preserved, because they have been recovered from quarry breccia using explosives. |
Review Questions
- What is the difference between a “derived” versus an “ancestral” trait? Give an example of both, seen in Au. afarensis.
- Which of the paleoenvironment hypotheses have been used to describe early hominin diversity, and which have been used to describe bipedalism?
- Which anatomical features for bipedalism do we see in early hominins?
- Describe the dentition of gracile and robust australopithecines. What might these tell us about their diets?
- List the hominin species argued to be associated with stone tool technologies. Are you convinced of these associations? Why/why not?
Key Terms
Arboreal: Related to trees or woodland.
Aridification: Becoming increasingly arid or dry, as related to the climate or environment.
Aridity Hypothesis: The hypothesis that long-term aridification and expansion of savannah biomes were drivers in diversification in early hominin evolution.
Assemblage: A collection demonstrating a pattern. Often pertaining to a site or region.
Bipedalism: The locomotor ability to walk on two legs.
Breccia: Hard, calcareous sedimentary rock.
Canines: The pointy teeth just next to the incisors, in the front of the mouth.
Cheek teeth: Or hind dentition (molars and premolars).
Chronospecies: Species that are said to evolve into another species, in a linear fashion, over time.
Clade: A group of species or taxa with a shared common ancestor.
Cladistics: The field of grouping organisms into those with shared ancestry.
Context: As pertaining to palaeoanthropology, this term refers to the place where an artifact or fossil is found.
Cores: The remains of a rock that has been flaked or knapped.
Cusps: The ridges or “bumps” on the teeth.
Dental formula: A technique to describe the number of incisors, canines, premolars, and molars in each quadrant of the mouth.
Derived traits: Newly evolved traits that differ from those seen in the ancestor.
Diastema: A tooth gap between the incisors and canines.
Early Stone Age (ESA): The earliest-described archaeological period in which we start seeing stone-tool technology.
East African Rift System (EARS): This term is often used to refer to the Rift Valley, expanding from Malawi to Ethiopia. This active geological structure is responsible for much of the visibility of the paleoanthropological record in East Africa.
Enamel: The highly mineralized outer layer of the tooth.
Encephalization: Expansion of the brain.
Extant: Currently living—i.e., not extinct.
Fallback foods: Foods that may not be preferred by an animal (e.g., foods that are not nutritionally dense) but that are essential for survival in times of stress or scarcity.
Fauna: The animals of a particular region, habitat, or geological period.
Faunal assemblages: Collections of fossils of the animals found at a site.
Faunal turnover: The rate at which species go extinct and are replaced with new species.
Flake: The piece knocked off of a stone core during the manufacture of a tool, which may be used as a stone tool.
Flora: The plants of a particular region, habitat, or geological period.
Folivorous: Foliage-eating.
Foramen magnum: The large hole (foramen) at the base of the cranium, through which the spinal cord enters the skull.
Fossil: The remains or impression of an organism from the past.
Frugivorous: Fruit-eating.
Generalist: A species that can thrive in a wide variety of habitats and can have a varied diet.
Glacial: Colder, drier periods during an ice age when there is more ice trapped at the poles.
Gracile: Slender, less rugged, or pronounced features.
Hallux: The big toe.
Holotype: A single specimen from which a species or taxon is described or named.
Hominin: A primate category that includes humans and our fossil relatives since our divergence from extant great apes.
Honing P3: The mandibular premolar alongside the canine (in primates, the P3), which is angled to give space for (and sharpen) the upper canines.
Hyper-robust: Even more robust than considered normal in the Paranthropus genus.
Hypodigm: A sample (here, fossil) from which researchers extrapolate features of a population.
Incisiform: An adjective referring to a canine that appears more incisor-like in morphology.
Incisors: The teeth in the front of the mouth, used to bite off food.
Interglacial: A period of milder climate in between two glacial periods.
Isotopes: Two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons, giving them the same chemical properties but different atomic masses.
Knappers: The people who fractured rocks in order to manufacture tools.
Knapping: The fracturing of rocks for the manufacture of tools.
Large Cutting Tool (LCT): A tool that is shaped to have functional edges.
Last Common Ancestor (LCA): The hypothetical final ancestor (or ancestral population) of two or more taxa before their divergence.
Lithic: Relating to stone (here to stone tools).
Lumbar lordosis: The inward curving of the lower (lumbar) parts of the spine. The lower curve in the human S-shaped spine.
Lumpers: Researchers who prefer to lump variable specimens into a single species or taxon and who feel high levels of variation is biologically real.
Megadont: An organism with extremely large dentition compared with body size.
Metacarpals: The long bones of the hand that connect to the phalanges (finger bones).
Molars: The largest, most posterior of the hind dentition.
Monophyletic: A taxon or group of taxa descended from a common ancestor that is not shared with another taxon or group.
Morphology: The study of the form or size and shape of things; in this case, skeletal parts.
Mosaic evolution: The concept that evolutionary change does not occur homogeneously throughout the body in organisms.
Obligate bipedalism: Where the primary form of locomotion for an organism is bipedal.
Occlude: When the teeth from the maxilla come into contact with the teeth in the mandible.
Oldowan: Lower Paleolithic, the earliest stone tool culture.
Orthognathic: The face below the eyes is relatively flat and does not jut out anteriorly.
Paleoanthropologists: Researchers that study human evolution.
Paleoenvironment: An environment from a period in the Earth’s geological past.
Parabolic: Like a parabola (parabola-shaped).
Phalanges: Long bones in the hand and fingers.
Phylogenetics: The study of phylogeny.
Phylogeny: The study of the evolutionary relationships between groups of organisms.
Pliocene: A geological epoch between the Miocene and Pleistocene.
Polytypic: In reference to taxonomy, having two or more group variants capable of interacting and breeding biologically but having morphological population differences.
Postcranium: The skeleton below the cranium (head).
Premolars: The smallest of the hind teeth, behind the canines.
Procumbent: In reference to incisors, tilting forward.
Prognathic: In reference to the face, the area below the eyes juts anteriorly.
Quaternary Ice Age: The most recent geological time period, which includes the Pleistocene and Holocene Epochs and which is defined by the cyclicity of increasing and decreasing ice sheets at the poles.
Relative dating: Dating techniques that refer to a temporal sequence (i.e., older or younger than others in the reference) and do not estimate actual or absolute dates.
Robust: Rugged or exaggerated features.
Site: A place in which evidence of past societies/species/activities may be observed through archaeological or paleontological practice.
Specialist: A specialist species can thrive only in a narrow range of environmental conditions or has a limited diet.
Splitters: Researchers who prefer to split a highly variable taxon into multiple groups or species.
Taxa: Plural of taxon, a taxonomic group such as species, genus, or family.
Taxonomy: The science of grouping and classifying organisms.
Techno-complex: A term encompassing multiple assemblages that share similar traits in terms of artifact production and morphology.
Thermoregulation: Maintaining body temperature through physiologically cooling or warming the body.
Ungulates: Hoofed mammals—e.g., cows and kudu.
Volcanic tufts: Rock made from ash from volcanic eruptions in the past.
Valgus knee: The angle of the knee between the femur and tibia, which allows for weight distribution to be angled closer to the point above the center of gravity (i.e., between the feet) in bipeds.
About the Authors

Kerryn Warren, Ph.D.
Grad Coach International, kerryn.warren@gmail.com
Kerryn Warren is a dissertation coach at Grad Coach International and is passionate about stimulating research thinking in students of all levels. She has lectured on multiple topics, including archaeology and human evolution, with her research and science communication interests including hybridization in the hominin fossil record (stemming from research from her Ph.D.) and understanding how evolution is taught in South African schools. She also worked as one of the “Underground Astronauts,” selected to excavate Homo naledi remains from the Rising Star Cave System in the Cradle of Humankind.

K. Lindsay Hunter, M.A., Ph.D. candidate
CARTA, k.lindsay.hunter@gmail.com
Lindsay Hunter is a trained palaeoanthropologist who uses her more than 15 years of experience to make sense of the distant past of our species to build a better future. She received her master’s degree in biological anthropology from the University of Iowa and is completing her Ph.D. in archaeology at the University of the Witwatersrand in Johannesburg, South Africa. She has studied fossil and human bone collections across five continents with major grant support from the National Science Foundation (United States) and the Wenner-Gren Foundation for Anthropological Research. As a National Geographic Explorer, Lindsay developed and managed the National Geographic–sponsored Umsuka Public Palaeoanthropology Project in the Cradle of Humankind World Heritage Site (CoH WHS) in South Africa from within Westbury Township, Johannesburg, between 2016–2019. She currently serves as the Community Engagement & Advancement Director for CARTA: The UC San Diego/Salk Institute Center for Academic Research and Training in Anthropogeny in La Jolla, California.

Navashni Naidoo, M.Sc.
University of Cape Town, nnaidoo2@illinois.edu
Navashni Naidoo is a researcher at Nelson Mandela University, lecturing on physical geology. She completed her Master’s in Science in Archaeology in 2017 at the University of Cape Town. Her research interests include developing paleoenvironmental proxies suited to the African continent, behavioral ecology, and engaging with community-driven archaeological projects. She has excavated at Stone Age sites across Southern Africa and East Africa. Navashni is currently pursuing a PhD in the Department of Anthropology at the University of Illinois.

Silindokuhle Mavuso, M.Sc.
University of Witwatersrand, S.muvaso@ru.ac.za
Silindokuhle has always been curious about the world around him and how it has been shaped. He is a lecturer at Rhodes University of Witwatersrand (Wits), and conducts research on palaeoenvironmental reconstruction and change of the northeastern Turkana Basin’s Pleistocene sequence. Silindokuhle began his education with a B.Sc. (Geology, Archaeology, and Environmental and Geographical Sciences) from the University of Cape Town before moving to Wits for a B.Sc. Honors (geology and paleontology) and M.Sc. in geology. He is currently concluding his PhD Studies. During this time, he has gained more training as a Koobi Fora Fieldschool fellow (Kenya) as well as an Erasmus Mundus scholar (France). Silindokuhle is a Plio-Pleistocene geologist with a specific focus on identifying and explaining past environments that are associated with early human life and development through time. He is interested in a wide range of disciplines such as micromorphology, sedimentology, geochemistry, geochronology, and sequence stratigraphy. He has worked with teams from significant eastern and southern African hominid sites including Elandsfontein, Rising Star, Sterkfontein, Gondolin, Laetoli, Olduvai, and Koobi Fora.
For Further Exploration
The Smithsonian Institution website hosts descriptions of fossil species, an interactive timeline, and much more.
The Maropeng Museum website hosts a wealth of information regarding South African Fossil Bearing sites in the Cradle of Humankind.
This quick comparison between Homo naledi and Australopithecus sediba from the Perot Museum.
This explanation of the braided stream by the Perot Museum.
A collation of 3-D files for visualizing (or even 3-D printing) for homes, schools, and universities.
PBS learning materials, including videos and diagrams of the Laetoli footprints, bipedalism, and fossils.
A wealth of information from the Australian Museum website, including species descriptions, family trees, and explanations of bipedalism and diet.
References
Alemseged, Zeresenay, Fred Spoor, William H. Kimbel, René Bobe, Denis Geraads, Denné Reed, and Jonathan G. Wynn. 2006. “A Juvenile Early Hominin Skeleton from Dikika, Ethiopia.” Nature 443 (7109): 296–301.
Asfaw, Berhane, Tim White, Owen Lovejoy, Bruce Latimer, Scott Simpson, and Gen Suwa. 1999. “Australopithecus garhi: A New Species of Early Hominid from Ethiopia.” Science 284 (5414): 629–635.
Behrensmeyer, Anna K., Nancy E. Todd, Richard Potts, and Geraldine E. McBrinn. 1997. “Late Pliocene Faunal Turnover in the Turkana Basin, Kenya, and Ethiopia.” Science 278 (5343): 637–640.
Berger, Lee R., Darryl J. De Ruiter, Steven E. Churchill, Peter Schmid, Kristian J. Carlson, Paul HGM Dirks, and Job M. Kibii. 2010. “Australopithecus sediba: A New Species of Homo-like Australopith from South Africa.” Science 328 (5975): 195–204.
Bobe, René, and Anna K. Behrensmeyer. 2004. “The Expansion of Grassland Ecosystems in Africa in Relation to Mammalian Evolution and the Origin of the Genus Homo.” Palaeogeography, Palaeoclimatology, Palaeoecology 207 (3–4): 399–420.
Brain, C. K. 1967. “The Transvaal Museum’s Fossil Project at Swartkrans.” South African Journal of Science 63 (9): 378–384.
Broom, R. 1938a. “More Discoveries of Australopithecus.” Nature 141 (1): 828–829.
Broom, R. 1938b. “The Pleistocene Anthropoid Apes of South Africa.” Nature 142 (3591): 377–379.
Broom, R. 1947. “Discovery of a New Skull of the South African Ape-Man, Plesianthropus.” Nature 159 (4046): 672.
Broom, R. 1950. “The Genera and Species of the South African Fossil Ape-Man.” American Journal of Physical Anthropology 8 (1): 1–14.
Brunet, Michel, Alain Beauvilain, Yves Coppens, Emile Heintz, Aladji HE Moutaye, and David Pilbeam. 1995. “The First Australopithecine 2,500 Kilometers West of the Rift Valley (Chad).” Nature 378 (6554): 275–273.
Cerling, Thure E., Jonathan G. Wynn, Samuel A. Andanje, Michael I. Bird, David Kimutai Korir, Naomi E. Levin, William Mace, Anthony N. Macharia, Jay Quade, and Christopher H. Remien. 2011. “Woody Cover and Hominin Environments in the Past 6 Million Years.” Nature 476, no. 7358 (2011): 51-56..
Clarke, Ronald J. 1998. “First Ever Discovery of a Well-Preserved Skull and Associated Skeleton of Australopithecus.” South African Journal of Science 94 (10): 460–463.
Clarke, Ronald J. 2013. “Australopithecus from Sterkfontein Caves, South Africa.” In The Paleobiology of Australopithecus, edited by K. E. Reed, J. G. Fleagle, and R. E. Leakey, 105–123. Netherlands: Springer.
Clarke, Ronald J., and Kathleen Kuman. 2019. “The Skull of StW 573, a 3.67 Ma Australopithecus Prometheus Skeleton from Sterkfontein Caves, South Africa.” Journal of Human Evolution 134: 102634.
Clarke, R. J., and P. V. Tobias. 1995. “Sterkfontein Member 2 Foot Bones of the Oldest South African Hominid.” Science 269 (5223): 521–524.
Constantino, P. J., and B. A. Wood. 2004. “Paranthropus Paleobiology”. In Miscelanea en Homenae a Emiliano Aguirre, volumen III: Paleoantropologia, edited by E. G. Pérez and S. R. Jara, 136–151. Alcalá de Henares: Museo Arqueologico Regional.
Constantino, P. J., and B. A. Wood. 2007. “The Evolution of Zinjanthropus boisei.” Evolutionary Anthropology: Issues, News, and Reviews 16 (2): 49–62.
Dart, Raymond A. 1925. “Australopithecus africanus, the Man-Ape of South Africa.” Nature 115: 195–199.
Darwin, Charles. 1871. The Descent of Man: And Selection in Relation to Sex. London: J. Murray.
Daver, Guillaume, F. Guy, Hassane Taïsso Mackaye, Andossa Likius, J-R. Boisserie, Abderamane Moussa, Laurent Pallas, Patrick Vignaud, and Nékoulnang D. Clarisse. 2022. “Postcranial Evidence of Late Miocene Hominin Bipedalism in Chad.” Nature 609 (7925): 94–100.
Heinzelin, Jean de, J. Desmond Clark, Tim White, William Hart, Paul Renne, Giday WoldeGabriel, Yonas Beyene, and Elisabeth Vrba. 1999. “Environment and Behavior of 2.5-Million-Year-Old Bouri Hominids.” Science 284 (5414): 625–629.
DeMenocal, Peter B. D. 2004. “African Climate Change and Faunal Evolution during the Pliocene–Pleistocene.” Earth and Planetary Science Letters 220 (1–2): 3–24.
DeMenocal, Peter B. D. and J. Bloemendal, J. 1995. “Plio-Pleistocene Climatic Variability in Subtropical Africa and the Paleoenvironment of Hominid Evolution: A Combined Data-Model Approach.” In Paleoclimate and Evolution, with Emphasis on Human Origins, edited by E. S. Vrba, G. H. Denton, T. C. Partridge, and L. H. Burckle, 262–288. New Haven: Yale University Press.
Dirks, Paul HGM, Job M. Kibii, Brian F. Kuhn, Christine Steininger, Steven E. Churchill, Jan D. Kramers, Robyn Pickering, Daniel L. Farber, Anne-Sophie Mériaux, Andy I. R. Herries, Geoffrey C. P. King, And Lee R. Berger. 2010. “Geological Setting and Age of Australopithecus sediba from Southern Africa.” Science 328 (5975): 205–208.
Faith, J. Tyler, and Anna K. Behrensmeyer. 2013. “Climate Change and Faunal Turnover: Testing the Mechanics of the Turnover-Pulse Hypothesis with South African Fossil Data.” Paleobiology 39 (4): 609–627.
Grine, Frederick E. 1988. “New Craniodental Fossils of Paranthropus from the Swartkrans Formation and Their Significance in ‘Robust’ Australopithecine Evolution.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 223–243. New York: Aldine de Gruyter.
Grine, Frederick E., Carrie S. Mongle, John G. Fleagle, and Ashley S. Hammond. 2022. “The Taxonomic Attribution of African Hominin Postcrania from the Miocene through the Pleistocene: Associations and Assumptions.” Journal of Human Evolution 173: 103255.
Haile-Selassie, Yohannes, Luis Gibert, Stephanie M. Melillo, Timothy M. Ryan, Mulugeta Alene, Alan Deino, Naomi E. Levin, Gary Scott, and Beverly Z. Saylor. 2015. “New Species from Ethiopia Further Expands Middle Pliocene Hominin Diversity.” Nature 521 (7553): 432–433.
Haile-Selassie, Yohannes, Stephanie M. Melillo, Antonino Vazzana, Stefano Benazzi, and Timothy M. Ryan. 2019. “A 3.8-Million-Year-Old Hominin Cranium from Woranso-Mille, Ethiopia.” Nature 573 (7773): 214-219.
Harmand, Sonia, Jason E. Lewis, Craig S. Feibel, Christopher J. Lepre, Sandrine Prat, Arnaud Lenoble, Xavier Boës et al. 2015. “3.3-Million-Year-Old Stone Tools from Lomekwi3, West Turkana, Kenya.” Nature 521 (7552): 310–316.
Hay, Richard L. 1990. “Olduvai Gorge: A Case History in the Interpretation of Hominid Paleoenvironments.” In East Africa: Establishment of a Geologic Framework for Paleoanthropology, edited by L. Laporte, 23–37. Boulder: Geological Society of America.
Hay, Richard L., and Mary D. Leakey. 1982. “The Fossil Footprints of Laetoli.” Scientific American 246 (2): 50–57.
Hlazo, Nomawethu. 2015. “Paranthropus: Variation in Cranial Morphology.” Honours thesis, Archaeology Department, University of Cape Town, Cape Town.
Hlazo, Nomawethu. 2018. “Variation and the Evolutionary Drivers of Diversity in the Genus Paranthropus.” Master’s thesis, Archaeology Department, University of Cape Town, Cape Town.
Johanson, D. C., T. D. White, and Y. Coppens. 1978. “A New Species of the Genus Australopithecus (Primates: Hominidae) from the Pliocene of East Africa.” Kirtlandia 28: 1–14.
Kimbel, William H. 2015. “The Species and Diversity of Australopiths.” In Handbook of Paleoanthropology, 2nd ed., edited by T. Hardt, 2071–2105. Berlin: Springer.
Kimbel, William H., and Lucas K. Delezene. 2009. “‘Lucy’ Redux: A Review of Research on Australopithecus afarensis.” American Journal of Physical Anthropology 140 (S49): 2–48.
Kingston, John D. 2007. “Shifting Adaptive Landscapes: Progress and Challenges in Reconstructing Early Hominid Environments.” American Journal of Physical Anthropology 134 (S45): 20–58.
Kingston, John D., and Terry Harrison. 2007. “Isotopic Dietary Reconstructions of Pliocene Herbivores at Laetoli: Implications for Early Hominin Paleoecology.” Palaeogeography, Palaeoclimatology, Palaeoecology 243 (3–4): 272–306.
Leakey, Louis S. B. 1959. “A New Fossil Skull from Olduvai.” Nature 184 (4685): 491–493.
Leakey, Mary 1971. Olduvai Gorge, Vol. 3. Cambridge: Cambridge University Press.
Leakey, Mary D., and Richard L. Hay. 1979. “Pliocene Footprints in the Laetoli Beds at Laetoli, Northern Tanzania.” Nature 278 (5702): 317–323.
Leakey, Meave G., Craig S. Feibel, Ian McDougall, and Alan Walker. 1995. “New Four–Million-Year-Old Hominid Species from Kanapoi and Allia Bay, Kenya.” Nature 376 (6541): 565–571.
Meave G., Fred Spoor, Frank H. Brown, Patrick N. Gathogo, Christopher Kiarie, Louise N. Leakey, and Ian McDougall. 2001. “New Hominin Genus from Eastern Africa Shows Diverse Middle Pliocene Lineages.” Nature 410 (6827): 433–440.
Lebatard, Anne-Elisabeth, Didier L. Bourlès, Philippe Duringer, Marc Jolivet, Régis Braucher, Julien Carcaillet, Mathieu Schuster et al. 2008. “Cosmogenic Nuclide Dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene Hominids from Chad.” Proceedings of the National Academy of Sciences 105 (9): 3226–3231.
Lee-Thorp, Julia. 2011. “The Demise of ‘Nutcracker Man.’” Proceedings of the National Academy of Sciences 108 (23): 9319–9320.
Lombard, Marlize, L. Y. N. Wadley, Janette Deacon, Sarah Wurz, Isabelle Parsons, Moleboheng Mohapi, Joane Swart, and Peter Mitchell. 2012. “South African and Lesotho Stone Age Sequence Updated.” The South African Archaeological Bulletin 67 (195): 123–144.
Maslin, Mark A., Chris M. Brierley, Alice M. Milner, Susanne Shultz, Martin H. Trauth, and Katy E. Wilson. 2014. “East African Climate Pulses and Early Human Evolution.” Quaternary Science Reviews 101: 1–17.
McHenry, Henry M. 2009. “Human Evolution.” In Evolution: The First Four Billion Years, edited by M. Ruse and J. Travis, 256–280. Cambridge: The Belknap Press of Harvard University Press..
Patterson, Bryan, and William W. Howells. 1967. “Hominid Humeral Fragment from Early Pleistocene of Northwestern Kenya.” Science 156 (3771): 64–66.
Pickering, Robyn, and Jan D. Kramers. 2010. “Re-appraisal of the Stratigraphy and Determination of New U-Pb Dates for the Sterkfontein Hominin Site.” Journal of Human Evolution 59 (1): 70–86.
Potts, Richard. 1998. “Environmental Hypotheses of Hominin Evolution.” American Journal of Physical Anthropology 107 (S27): 93–136.
Potts, Richard. 2013. “Hominin Evolution in Settings of Strong Environmental Variability.” Quaternary Science Reviews 73: 1–13.
Rak, Yoel. 1983. The Australopithecine Face. New York: Academic Press.
Rak, Yoel. 1988. “On Variation in the Masticatory System of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by M. Ruse and J. Travis, 193–198. New York: Aldine de Gruyter.
Semaw, Sileshi. 2000. “The World’s Oldest Stone Artefacts from Gona, Ethiopia: Their Implications for Understanding Stone Technology and Patterns of Human Evolution between 2.6 Million Years Ago and 1.5 Million Years Ago.” Journal of Archaeological Science 27(12): 1197–1214.
Shipman, Pat. 2002. The Man Who Found the Missing Link: Eugene Dubois and his Lifelong Quest to Prove Darwin Right. New York: Simon & Schuster.
Spoor, Fred. 2015. “Palaeoanthropology: The Middle Pliocene Gets Crowded.” Nature 521 (7553): 432–433.
Strait, David S., Frederick E. Grine, and Marc A. Moniz. 1997. A Reappraisal of Early Hominid Phylogeny.” Journal of Human Evolution 32 (1): 17–82.
Thackeray, J. Francis. 2000. “‘Mrs. Ples’ from Sterkfontein: Small Male or Large Female?” The South African Archaeological Bulletin 55: 155–158.
Thackeray, J. Francis, José Braga, Jacques Treil, N. Niksch, and J. H. Labuschagne. 2002. “‘Mrs. Ples’ (Sts 5) from Sterkfontein: An Adolescent Male?” South African Journal of Science 98 (1–2): 21–22.
Toth, Nicholas. 1985. “The Oldowan Reassessed.” Journal of Archaeological Science 12 (2): 101–120.
Vrba, E. S. 1988. “Late Pliocene Climatic Events and Hominid Evolution.” In The Evolutionary History of the Robust Australopithecines, edited by F. E. Grine, 405–426. New York: Aldine.
Vrba, Elisabeth S. 1998. “Multiphasic Growth Models and the Evolution of Prolonged Growth Exemplified by Human Brain Evolution.” Journal of Theoretical Biology 190 (3): 227–239.
Vrba, Elisabeth S. 2000. “Major Features of Neogene Mammalian Evolution in Africa.” In Cenozoic Geology of Southern Africa, edited by T. C. Partridge and R. Maud, 277–304. Oxford: Oxford University Press.
Walker, Alan C., and Richard E. Leakey. 1988. “The Evolution of Australopithecus boisei.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 247–258. New York: Aldine de Gruyter.
Walker, Alan, Richard E. Leakey, John M. Harris, and Francis H. Brown. 1986. “2.5-my Australopithecus boisei from West of Lake Turkana, Kenya.” Nature 322 (6079): 517–522.
Ward, Carol, Meave Leakey, and Alan Walker. 1999. “The New Hominid Species Australopithecus anamensis.” Evolutionary Anthropology 7 (6): 197–205.
White, Tim D. 1988. “The Comparative Biology of ‘Robust’ Australopithecus: Clues from Content.” In Evolutionary History of the “Robust” Australopithecines, edited by F. E. Grine, 449–483. New York: Aldine de Gruyter.
White, Tim D., Gen Suwa, and Berhane Asfaw. 1994. “Australopithecus ramidus, a New Species of Early Hominid from Aramis, Ethiopia.” Nature 371 (6495): 306–312.
Wood, Bernard. 2010. “Reconstructing Human Evolution: Achievements, Challenges, and Opportunities.” Proceedings of the National Academy of Sciences 10 (2): 8902–8909.
Wood, Bernard, and Eve K. Boyle. 2016. “Hominin Taxic Diversity: Fact or Fantasy?” Yearbook of Physical Anthropology 159 (S61): 37–78.
Wood, Bernard, and Kes Schroer. 2017. “Paranthropus: Where Do Things Stand?” In Human Paleontology and Prehistory, edited by A. Marom and E. Hovers, 95–107. New York: Springer, Cham.
Acknowledgements
All of the authors in this section are students and early career researchers in paleoanthropology and related fields in South Africa (or at least have worked in South Africa). We wish to thank everyone who supports young and diverse talent in this field and would love to further acknowledge Black, African, and female academics who have helped pave the way for us.
Walking on two legs.
A primate category that includes humans and our fossil relatives since our divergence from extant great apes.
The hypothetical final ancestor (or ancestral population) of two or more taxa before their divergence.
The remains or impression of an organism from the past.
Researchers that study human evolution.
Expansion of the brain.
The study of the form or size and shape of things; in this case, skeletal parts.
This term is often used to refer to the Rift Valley, expanding from Malawi to Ethiopia. This active geological structure is responsible for much of the visibility of the paleoanthropological record in East Africa.
A place in which evidence of past societies/species/activities may be observed through archaeological or paleontological practice.
The science of grouping and classifying organisms.
The study of the evolutionary relationships between groups of organisms.
Plural of taxon, a taxonomic group such as species, genus, or family.
The field of grouping organisms into those with shared ancestry.
The study of phylogeny.
A trait that has been recently modified, most helpful when assigning taxonomic classification.
A grouping based on ancestral relationships; a branch of the evolutionary tree.
Researchers who prefer to lump variable specimens into a single species or taxon and who feel high levels of variation is biologically real.
Researchers who prefer to split a highly variable taxon into multiple groups or species.
In reference to taxonomy, having two or more group variants capable of interacting and breeding biologically but having morphological population differences.
Species that are said to evolve into another species, in a linear fashion, over time.
The animals of a particular region, habitat, or geological period.
An environment from a period in the Earth’s geological past.
Collections of fossils of the animals found at a site.
Two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons, giving them the same chemical properties but different atomic masses.
The plants of a particular region, habitat, or geological period.
Related to trees or woodland.
The hypothesis that long-term aridification and expansion of savannah biomes were drivers in diversification in early hominin evolution.
Becoming increasingly arid or dry, as related to the climate or environment.
Hoofed mammals—e.g., cows and kudu.
A specialist species can thrive only in a narrow range of environmental conditions or has a limited diet.
A species that can thrive in a wide variety of habitats and can have a varied diet.
The rate at which species go extinct and are replaced with new species.
The most recent geological time period, which includes the Pleistocene and Holocene Epochs and which is defined by the cyclicity of increasing and decreasing ice sheets at the poles.
A period of milder climate in between two glacial periods.
Colder, drier periods during an ice age when there is more ice trapped at the poles.
Where the primary form of locomotion for an organism is bipedal.
Currently living—i.e., not extinct.
The skeleton below the cranium (head).
The concept that evolutionary change does not occur homogeneously throughout the body in organisms.
The big toe.
Or hind dentition (molars and premolars).
A geological epoch between the Miocene and Pleistocene.
Fruit-eating.
Foliage-eating.
The face below the eyes is relatively flat and does not jut out anteriorly.
A technique to describe the number of incisors, canines, premolars, and molars in each quadrant of the mouth.
The spatula-shaped teeth at the front of the mouth.
In most primates, these are the longest of the teeth, often conical in shape and used as a weapon against predators or others of their species.
The smallest of the hind teeth, behind the canines.
The largest teeth at the back of the mouth; used for chewing. In primates, these teeth usually have between three and five cusps.
When the teeth from the maxilla come into contact with the teeth in the mandible.
In reference to incisors, tilting forward.
An adjective referring to a canine that appears more incisor-like in morphology.
A space between the teeth, usually for large canines to fit when the mouth is closed.
The mandibular premolar alongside the canine (in primates, the P3), which is angled to give space for (and sharpen) the upper canines.
Like a parabola (parabola-shaped).
The highly mineralized outer layer of the tooth.
The bumps on the chewing surface of the premolars and molars, which can be quite sharp in some species.
A single specimen from which a species or taxon is described or named.
Slender, less rugged, or pronounced features.
Rugged or exaggerated features.
Rock made from ash from volcanic eruptions in the past.
As pertaining to palaeoanthropology, this term refers to the place where an artifact or fossil is found.
In reference to the face, the area below the eyes juts anteriorly.
Dating techniques that refer to a temporal sequence (i.e., older or younger than others in the reference) and do not estimate actual or absolute dates.
Foods that may not be preferred by an animal (e.g., foods that are not nutritionally dense) but that are essential for survival in times of stress or scarcity.
A taxon or group of taxa descended from a common ancestor that is not shared with another taxon or group.
A sample (here, fossil) from which researchers extrapolate features of a population.
Even more robust than considered normal in the Paranthropus genus.
The earliest-described archaeological period in which we start seeing stone-tool technology.
Relating to stone (here to stone tools).
The fracturing of rocks for the manufacture of tools.
A term encompassing multiple assemblages that share similar traits in terms of artifact production and morphology.
A collection demonstrating a pattern. Often pertaining to a site or region.
The people who fractured rocks in order to manufacture tools.
The piece knocked off of a stone core during the manufacture of a tool, which may be used as a stone tool.
A tool that is shaped to have functional edges.
Lower Paleolithic, the earliest stone tool culture.
