11 Archaic Homo
Amanda Wolcott Paskey, M.A., Cosumnes River College
AnnMarie Beasley Cisneros, M.A., American River College
This chapter is a revision from “Chapter 11: Archaic Homo” by Amanda Wolcott Paskey and AnnMarie Beasley Cisneros. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Identify the main groupings of Archaic Homo sapiens, such as Neanderthals.
- Explain how shifting environmental conditions required flexibility of adaptations, both anatomically and behaviorally.
- Describe the unique anatomical and cultural characteristics of Archaic Homo sapiens, including Neanderthals, in contrast to other hominins.
- Articulate how Middle Pleistocene hominin fossils fit into evolutionary trends including cranial capacity (brain size) development, cultural innovations, and migration patterns.
- Identify the shared traits, regional variations, and local adaptations among Archaic Homo sapiens.
- Detail the increased complexity and debates surrounding the classification of hominins in light of transitional species, species admixture, etc.
Breaking the Stigma of the “Caveman”
What do you think of when you hear the word “caveman”? Perhaps you imagine a character from a film such as The Croods, Tarzan, and Encino Man or from the cartoon The Flintstones. Maybe you picture the tennis-playing, therapy-going hairy Neanderthals from Geico Insurance commercials. Or perhaps you imagine characters from The Far Side or B.C. comics. Whichever you picture, the character in your mind is likely stooped over with a heavy brow, tangled long locks and other body hair, and clothed in animal skins, if anything. They might be holding a club with a confused look on their face, standing at the entrance to a cave or dragging an animal carcass to a fire for their next meal (see Figure 11.1). You might have even signed up to take this course because of what you knew—or expected to learn—about “cavemen.”

These images have long been the stigma and expectation about our ancestors at the transition to modern Homo sapiens. Tracing back to works as early as Carl Linnaeus, scientists once propagated and advanced this imagery, creating a clear picture in the minds of early scholars that informed the general public, even through today, that Archaic Homo sapiens, “cavemen,” were somehow fundamentally different and much less intelligent than we are now. Unfortunately, this view is overly simplistic, misleading, and incorrect. Understanding what Archaic Homo sapiens were actually like requires a much more complex and nuanced picture, one that comes into sharper focus as continuing research uncovers more about the lives of our not-too-distant (and not-too-different) ancestors.
The first characterizations of Archaic Homo sapiens were formed from limited fossil evidence in a time when ethnocentric and species-centric perspectives (anthropocentrism) were more widely accepted and entrenched in both society and science. Today, scientists are working from a more complete fossil record from three continents (Africa, Asia, and Europe), and genetic evidence informs their analyses and conclusions. The existence of Archaic Homo sapiens marks an exciting point in our lineage—a point at which many modern traits had emerged and key refinements were on the horizon. Anatomically, humans today are not that much different from Archaic Homo sapiens.
This chapter will examine how the environment with which Archaic Homo sapiens had to contend shaped their biological and cultural evolution. It will also examine the key anatomical traits that define this group of fossils, focusing on the most well-known of them, including Neanderthals. The chapter will describe cultural innovations that aided their adaptation to the changing environment, as well as their geographic distribution and regional variations. Additionally, exciting new research is examined that suggests even greater nuance and complexity during this time period.
The Changing Environment
While modern climate change is of critical concern today due to its cause (human activity) and pace (unprecedentedly rapid), the existence of global climate change itself is not a recent phenomenon. The focus of this chapter, the Middle Pleistocene (roughly between 780 kya and 125 kya), is the time period in which Archaic Homo sapiens appears in the fossil record—a time that witnessed some of the most drastic climatic changes in human existence. During this time period, there were 15 major and 50 minor glacial events in Europe, alone.
What exactly is glaciation? When scientists talk about glacial events, they are referring to the climate being in an ice age. This means that the ocean levels were much lower than today, because much of the earth’s water was tied up in large glaciers or ice sheets. Additionally, the average temperature would have been much cooler, which would have better supported an Arctic or tundra-adapted plant-and-animal ecosystem in northern latitudes. The most interesting and relevant features of Middle Pleistocene glacial events are the sheer number of them and their repeated bouts: this era alternated between glacial periods and warmer periods, known as interglacials. In other words, the planet wasn’t in an ice age the whole time.
You can see the dramatic and increasing fluctuations in temperature, recorded through foraminifera, in Figure 11.2. The distance between highs and lows demonstrates the severity of temperature shifts. Much as the Richter scale represents more intense earthquakes with more dramatic peaks, so too does this chart, which uses dramatic peaks to demonstrate intense temperature swings.
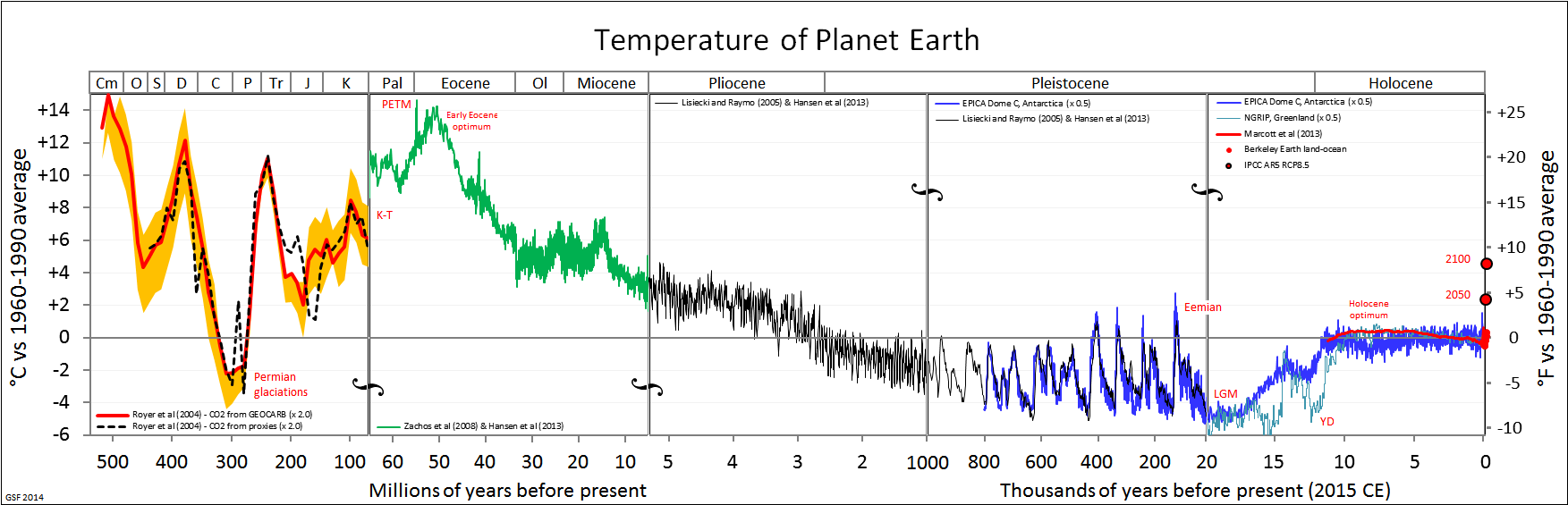
Glacial periods are defined by Earth’s average temperature being lower. Worldwide, temperatures are reduced, with cold areas becoming even colder. Huge portions of the landscape may have become inaccessible during glacial events due to the formation of glaciers and massive ice sheets. In Europe, the Scandinavian continental glacier covered what is today Ireland, England, Sweden, Norway, Denmark, and some of continental Europe. Plant and animal communities shifted to lower latitudes along the periphery of ice sheets. Additionally, some new land was opened during glacials. Evaporation with little runoff reduced sea levels by as much as almost 150 meters, shifting coastlines outward by in some instances as much as almost 100 kilometers. Additionally, land became exposed that connected what were previously unconnected continents such as Africa into Yemen at the Gulf of Aden.
Glacial periods also affected equatorial regions and other regions that are today thought of as warmer or at least more temperate parts of the globe, including Africa. While these areas were not covered with glaciers, increased global glaciation resulted in lower sea levels and expanded coastlines. Cooler temperatures were accompanied by the drying of the climate, which caused significantly reduced rainfall, increased aridity, and the expansion of deserts. It is an interesting question to consider whether the same plants and animals that lived in these regions prior to the ice ages would be able to survive and thrive in this new climate. Plant and animal communities shifted in response to the changing climate, whenever possible.
Surviving During the Middle Pleistocene
Rather than a single selective force, the Middle Pleistocene was marked by periods of fluctuation, not just cold periods. Interglacials interrupted glaciations, reversing trends in sea level, coastline, temperature, precipitation, and aridity, as well as glacier size and location. Interglacials are marked by increased rainfall and a higher temperature, which causes built-up ice in glaciers to melt. This leads to glacial retreat, which is the shrinking of glaciers and the movement of the glaciers back toward the poles, as we’ve seen in our lifetime. During interglacials, sea levels increase, flooding some previously exposed coastlines and continental connections. In addition, plant and animal communities shift accordingly, often finding more temperate climates to the north and less arid and more humid climates in the tropics (Van Andel and Tzedakis 1996).
Scientists have found that the Olorgesailie region in southern Kenya was at various times in the Middle Pleistocene a deep lake, a drought-dried lakebed with an area criss-crossed by small streams, and a grassland. While various animal species would have moved in and out of the area as the climate shifted, some animal species went extinct, and new, often related, species took up residence. The trend, scientists noted, was that animals with more specialized features went extinct and animals with more generalized features, such as animals we see today, survived in this changing climatic time period. For example, a zebra with specialized teeth for eating grass was ultimately replaced by a zebra that could eat both grass and other types of vegetation. If this small, localized example shows such a dramatic change in terms of the environment and the plant and animal biocommunities, what would have been the impact on humans?
There is no way humans could have escaped the effects of Middle Pleistocene climate change, no matter what region of the world they were living in. As noted earlier, and as evidenced by what was seen in the other biotic communities, humans would have faced changing food sources as previous sources of food may have gone extinct or moved to a different latitude. Depending on where they were living, fresh water may have been limited. Durial glacials, lower sea levels would have given humans more land to live on, while the interglacials would have reduced the available land through the increase in rainfall and associated sea level rise. Dry land connections between the continents would have made movement from one continent to another by foot easier at times than today, although these passageways were not consistently available through the Middle Pleistocene due to the glacial/interglacial cycle. Finally, as evidenced by the Olorgesailie region in Kenya, during the Middle Pleistocene animal species that were overly specialized to one particular type of environment were less likely to survive when compared to their more generalized counterparts. Evidence suggests that this same pattern may have held true for Archaic Homo sapiens, in terms of their ability to survive this dramatic period of climate change.
Defining Characteristics of Archaic Homo sapiens
Archaic Homo sapiens share our species name but are distinguished by the term “Archaic” as a way of recognizing both the long period of time between their appearance and ours, as well as the way in which human traits have continued to evolve over time—making Archaic Homo sapiens look slightly different from us today, despite being considered the same species. Living throughout Africa, and the Middle East during the Middle Pleistocene, Archaic Homo sapiens are considered, in many ways, transitional between Homo erectus and modern Homo sapiens (see Figure 11.3). Archaic Homo sapiens share the defining trait of an increased brain size of at least 1,100 cc and averaging 1,200 cc, although there are significant regional and temporal (time) variations. Because of these variations, scientists disagree on whether these fossils represent a single, variable species or multiple, closely related species (sometimes called Homo antecessor, Homo bodoensis, Homo heidelbergensis, Homo georgicus, Homo neanderthalensis, and Homo rhodesiensis).
An active area of scholarship in the discipline involves reconciling the diversity of species from this time period and establishing the phylogenetic relationships between them. The term “Archaic Homo sapiens” can mean different things to different scholars within the discipline. The intent of this chapter is to provide an understanding of the diversity of this time period and provide data used to make interpretations from among the most likely possibilities. Although we recognize that some anthropologists split many of these fossils into separate species, until the issue is resolved at the discipline level, this chapter will rely on the widely used naming conventions that refer to many fossils from this time period as Archaic Homo sapiens. We will discuss these contemporaneous fossils as a unit, with the exception of a particularly unique population living in Europe and West Asia known as the Neanderthals, which we will examine separately.
|
Trait |
Homo erectus |
Archaic Homo sapiens (including Neaderthals) |
Anatomically Modern Homo sapiens |
|---|---|---|---|
|
Time |
1.8 mya–200,000 ya |
600,000–40,000 ya |
315,000 ya–today |
|
Brain size |
900 cc |
1,200 cc (1,500 cc when including Neanderthals) |
1,400 cc |
|
Skull Shape |
Long and low, angular |
Intermediate |
Short and high, globular |
|
Forehead |
Absent |
Emerging |
Present |
|
Nasal Region |
Projecting nasal bones (bridge of the nose), no midfacial prognathism |
Wider nasal aperture and some midfacial prognathism, particularly pronounced among Neanderthals |
Narrower nasal aperture, no midfacial prognathism |
|
Chin |
Absent |
Absent |
Present |
|
Other Facial Features |
Large brow ridge and large projecting face |
Intermediate |
Small brow ridge and retracted face |
|
Other Skull Features |
Nuchal torus, sagittal keel, thick cranial bone |
Projecting occipital bone, often called occipital bun in Neanderthals; intermediate thickness of cranial bone |
Small bump on rear of skull, if anything; thin cranial bone |
|
Dentition |
Large teeth, especially front teeth |
Slightly smaller teeth; front teeth still large; retromolar gap in Neanderthals |
Smaller teeth |
|
Postcranial Features |
Robust bones of skeleton |
Robust bones of skeleton |
More gracile bones of skeleton |
When comparing Homo erectus, Archaic Homo sapiens, and anatomically modern Homo sapiens, one can see that Archaic Homo sapiens are intermediate in their physical form. For some features, this follows the trends first seen in Homo erectus with other features having early, less developed forms of traits seen in modern Homo sapiens. For example, Archaic Homo sapiens trended toward less angular and higher skulls than Homo erectus. However, the archaic skulls were not as short and globular and had less developed foreheads compared to anatomically modern Homo sapiens. Archaic Homo sapiens had smaller brow ridges and a less-projecting face than Homo erectus and slightly smaller teeth, although incisors and canines were often about as large as those of Homo erectus. Archaic Homo sapiens also had a wider nasal aperture, or opening for the nose, and a forward-projecting midfacial region, which is later seen more fully developed among Neanderthals and is known as midfacial prognathism. The occipital bone often projected and the cranial bone was of intermediate thickness, somewhat reduced from Homo erectus but not nearly as thin as that of anatomically modern Homo sapiens. The postcrania remained fairly robust. Identifying a set of features that is unique to Archaic Homo sapiens is a challenging task, due to both individual and geographic variation—these developments were not all present to the same degree in all individuals. Neanderthals are the exception, as they had several unique traits that made them notably different from modern Homo sapiens as well as their closely related Archaic cousins.
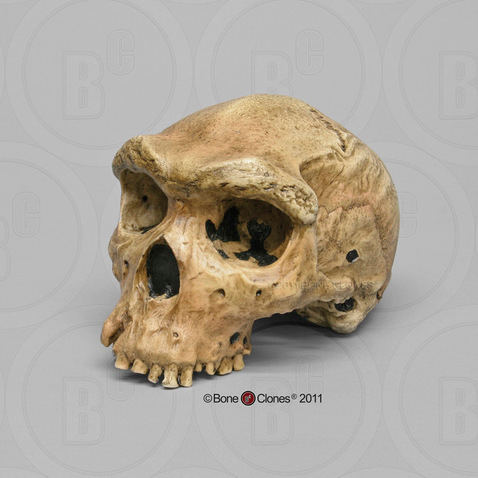
The one thing that is clear about Archaic Homo sapiens is that, despite general features, there is a lot of regional variation, which is first seen in the different Homo erectus specimens across Asia and Africa. While the general features of Archaic Homo sapiens, identified earlier, are present in the fossils of this time period, there are significant regional differences. The majority of this regional variation lies in the degree to which fossils have features more closely aligned with Homo erectus or with anatomically modern Homo sapiens.
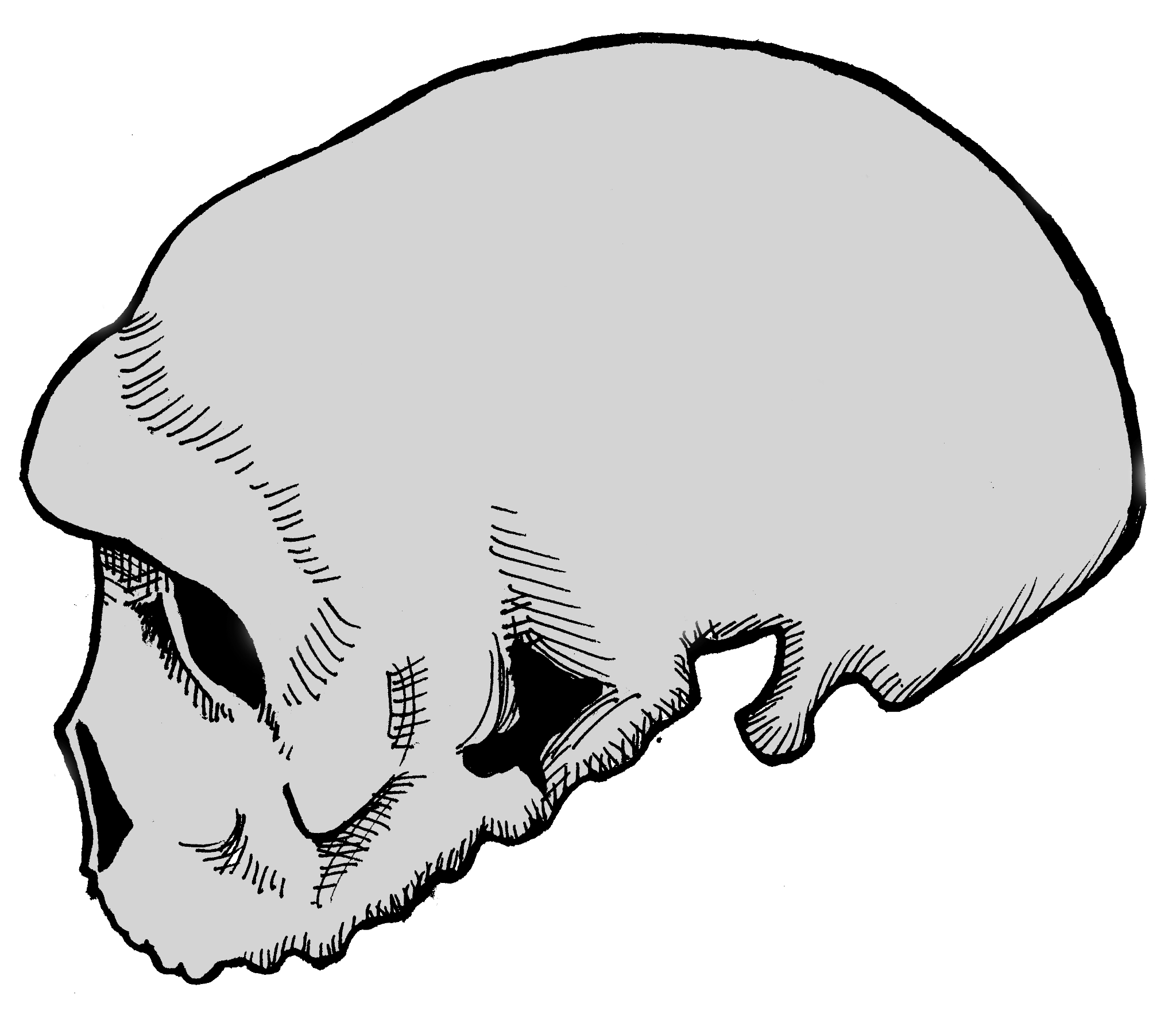
To illustrate this point, we will examine three exemplary specimens, one from each of the three continents on which Archaic Homo sapiens lived. First, in Africa, a specimen from Broken Hill is one of several individuals found in the Kabwe lead mine in Zambia. It had a large brain (1,300 cc) and taller cranium as well as many Homo erectus-like skull features, including massive brow ridges, a large face, and thick cranial bones (Figure 11.4). Second, one partial crania from Dali, China, is representative of Archaic Homo sapiens in Asia, with large and robust features with heavy brow ridges, akin to what is seen in Homo erectus, and a large cranial capacity intermediate between Homo erectus and anatomically modern Homo sapiens (Figure 11.5). Third, an almost-complete skeleton was found in northern Spain at Atapuerca. Atapuerca 5 (Figure 11.6) has thick cranial bone, an enlarged cranial capacity, intermediate cranial height, and a more rounded cranium than seen previously. Additionally, Atapuerca 5 demonstrates features that foreshadow Neanderthals, including increased midfacial prognathism. After examining some of the fossils such as those from Kabwe, Dali, and Atapuerca, the transitional nature of Archaic Homo sapiens is clear: their features place them squarely between Homo erectus and modern Homo sapiens.
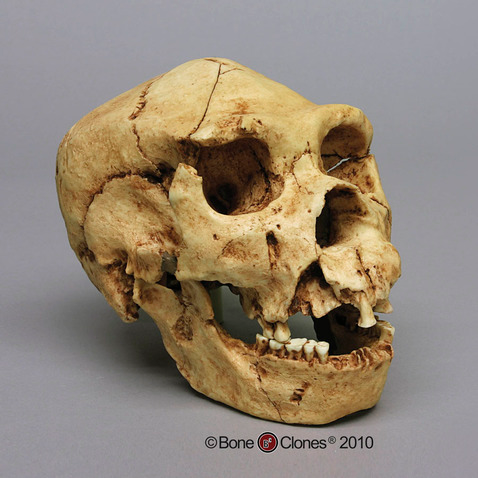
Due to the transitional nature of Archaic Homo sapiens, identifying the time period with which they are associated is problematic and complex. Generally, it is agreed that Archaic Homo sapiens lived between 600,000 and 200,000 years ago, with regional variation and overlap between Homo erectus on the early end of the spectrum and modern Homo sapiens and Neanderthals on the latter end. The earliest-known Archaic Homo sapiens fossils tentatively date to about 600,000 years ago in Africa, to around 300,000 years ago in Asia, and to about 350,000 years ago in Europe (and potentially as early as 600,000 years ago). Determining the end point of Archaic Homo sapiens is also problematic since it largely depends upon when the next subspecies of Homo sapiens appears and the classification of highly intermediate specimens. For example, in Africa, the end of Archaic Homo sapiens is met with the appearance of modern Homo sapiens, while in Europe it is the appearance of Neanderthals that is traditionally seen as marking the transition from other Archaic Homo sapiens.
It is important to remember that this time period is represented by many branching relationships and assuming an evolutionary trajectory that follows a single linear path would not be correct. Even still, Archaic Homo sapiens mark an important chapter in the human lineage, connecting more ancestral forms, such as Homo erectus, to modern Homo sapiens. During this period of climatic transition and fluctuation, Archaic Homo sapiens mirror the challenges of their environments. Showing increasing regional variation due to the need for local adaptation, there is no single archetype for this group; the defining characteristic seems to be variability.
Neanderthals
One well-known population of Archaic Homo sapiens are the Neanderthals, named after the site where they were first discovered in the Neander Valley, or “thal” in German, located near Dusseldorf, Germany. Popularly known as the stereotypical “cavemen” examined at the outset of this chapter, recent research is upending long-held beliefs about this group of Archaics. Neanderthal behavior was increasingly complex, far beyond what was exhibited by even other Archaic Homo sapiens discussed throughout this chapter. We implore you to forget the image of the iconic caveman and have an open mind when exploring the fossil evidence of the Neanderthals.
It is important to understand why Neanderthals are separated from other Archaic Homo sapiens. Unlike the rest of Archaic Homo sapiens, Neanderthals are easily defined and identified in many ways. Evidence suggests the time period when Neanderthals lived was between 150,000 and 40,000 years ago. There is a clear geographic boundary of where Neanderthals lived: western Europe, the Middle East, and western Asia. No Neanderthal fossils have ever been discovered outside of this area, including Africa. This is a bit curious, as other Archaics seem to have adapted in Africa and then migrated elsewhere, but Neanderthals’ regional association makes sense in light of the environment to which they were best adapted: namely, extreme cold weather. Additionally, Neanderthals have a unique and distinct cluster of physical characteristics. While a few aspects of Neanderthals are shared among some Archaic Homo sapiens, such as the types of tools, most Neanderthal anatomical and behavioral attributes are unique to them.
Neanderthals lived during some of the coldest times during the last Ice Age and at far northern latitudes. This means Neanderthals were living very close to the glacial edge, rather than in a more temperate region of the globe like some of their Archaic Homo sapiens relatives. While able to survive in arctic conditions, most Neanderthal sites dating to the glacial periods were found farther away from the severe cold, in a steppe tundra-like environment, which would have been more hospitable to Neanderthals, and their food sources, both flora and fauna (Ashton 2002; Nicholson 2017; Richter 2006).Their range likely expanded and contracted along with European glacial events, moving into the Middle East during glacial events when Europe became even cooler, and when the animals they hunted would have moved for the same reason. During interglacials, when Europe warmed a bit, Neanderthals and their prey would have been able to move back into Western Europe. Clearly, the true hallmark of Neanderthals is their adaptation to an unstable environment, shifting between warm and cold, as the climate was in constant flux throughout their existence (Adler et al. 2003; Boettger et al. 2009).
Many of the Neanderthals’ defining physical features are more extreme and robust versions of traits seen in other Archaic Homo sapiens, clustered in this single population. Brain size, namely an enlargement of the cranial capacity, is one such trait. The average Neanderthal brain size is around 1,500 cc, and the range for Neanderthal brains can extend to upwards of 1,700 cc. The majority of the increase in the brain occurs in the occipital region, or the back part of the brain, resulting in a skull that has a large cranial capacity with a distinctly long and low shape that is slightly wider than previous forms at the far back of the skull. Modern humans have a brain size comparable to that of Neanderthals; however, our brain expansion occurred in the frontal region of the brain, not the back, as in Neanderthal brains. This difference is also the main reason why Neanderthals lack the vertical forehead that modern humans possess. They simply did not need an enlarged forehead, because their brain expansion occurred in the rear of their brain. Due to cranial expansion, the back of the Neanderthal skull is less angular (as compared to Homo erectus), but not as rounded as Homo sapiens, producing an elongated shape, akin to a football.
Another feature that continues the trend noted in previous hominins is the enlargement of the nasal region, or the nose. Neanderthal noses are large and have a wide nasal aperture, which is the opening for the nose. While the nose is only made up of two bones, the nasals, the true size of the nose can be determined by looking at other facial features, including the nasal aperture, and the angle of the nasal and maxillary, or facial bones. In Neanderthals, these indicate a large, forward-projecting nose that appears to be pulled forward away from the rest of the face. This feature is further emphasized by the backward-sloping nature of the cheekbones, or the zygomatic arches. The unique shape and size of the Neanderthal nose is often characterized by the term midfacial prognathism—a jutting out of the middle portion of the face, or nose. This is in sharp contrast to the prognathism exhibited by other hominins, who exhibited prognathism, or the jutting out, of their jaws.
The teeth of the Neanderthals follow a similar pattern seen in the Archaic Homo sapiens, which is an overall reduction in size, especially as compared to the extremely large teeth seen in the genus Australopithecus. However, while the teeth continued to reduce, the jaw size did not keep pace, leaving Neanderthals with an oversized jaw for their teeth, and a gap between their final molar and the end of their jaw. This gap is called a retromolar gap.
The projecting occipital bone present in other Archaic Homo sapiens is also more prominent in Neanderthals, extending the trend found in Archaics. Among Neanderthals, this projection of bone is easily identified by its bun shape on the back of the skull and is known as an occipital bun. This projection appears quite similar to a dinner roll in size and shape. Its purpose, if any, remains unknown.
Continuing the Archaic Homo sapiens trend, Neanderthal brow ridges are prominent but somewhat smaller in size than those of Homo erectus and earlier Archaic Homo sapiens. In Neanderthals, the brow ridges are also often slightly less arched than those of other Archaic Homo sapiens.
In addition to extending traits present in Archaic Homo sapiens, Neanderthals possess several distinct traits. Neanderthal infraorbital foramina, the holes in the maxillae or cheek bones through which blood vessels pass, are notably enlarged compared to other hominins. The Neanderthal postcrania are also unique in that they demonstrate increased robusticity in terms of the thickness of bones and body proportions that show a barrel-shaped chest and short, stocky limbs, as well as increased musculature. These body portions are seen across the spectrum of Neanderthals—in men, women, and children.
Traditionally, many of the unique traits that Neanderthals possess were seen as adaptations to the extreme cold, dry environments in which they often lived and which exerted strong selective forces. For example, Bergmann’s and Allen’s Rules dictate that an increased body mass and short, stocky limbs are common in animals that live in cold conditions. Neanderthals were said to have matched the predictions of Bergmann’s and Allen’s Rules perfectly (Churchill 2006). In addition, the Neanderthal skull also exhibits adaptations to the cold. Neanderthals’ large infraorbital foramina allow for larger blood vessels, increasing the volume of blood that is found closest to the skin, which helps to keep the skin warmer. Their enlarged noses resulted in longer nasal passages and mucus membranes that warmed and moistened cold air before it reached the lungs. The Neanderthals’ larger nose has long been thought to have acted as a humidifier, easing physical exertion in their climate, although research on this particular trait continues to be studied and debated (Rae et al. 2011).
New research, however, seems to suggest that these unique skeletal adaptations might not have been strict adaptations to cold weather (Evteev et al. 2017; Pearce et al. 2013). For example, large brow ridges might have served as a way to shade the face from the sun. The increased occipital portion of the brain, some researchers state, was to support a larger visual system present in Neanderthals. This visual system would have given them increased light sensitivity, which would have been useful in higher latitudes that had dark winters. And, while recent modeling of nostril airflow on reconstructed Neanderthal specimens supports the notion that Neanderthals had extensive mucus membranes inside their noses, the data shows that modern Homo sapiens are superior to Neanderthals in our ability to use our noses as a way to warm and cool air. However, Neanderthals were able to snort air through their noses better than we can. Why is this important? When combined with the fact that Neanderthals tended to prefer a more temperate, tundra-like environment, and that other physical traits suggest that Neanderthals had huge bodies that needed massive amounts of calories to sustain them, the picture gets clearer. Massive amounts of energy would have been required to power a Neanderthal body, and anything that might have made them more calorically efficient would have been favored. Efficient breathing, through larger noses into large lungs, meaning deeper breaths, would have been favored. To further save energy expenditure, body sizes might have been sacrificed as well. These same types of adaptations are similar to ones seen in children today who are born in high altitudes, not cold climates. Figure 11.7 provides a summary of these unique features of the Neanderthal.
|
Distinct Neanderthal Anatomical Features |
|
|---|---|
|
Brain Size |
1,500 cc average |
|
Skull Shape |
Long and low |
|
Brow Ridge Size |
Large |
|
Nose Size |
Large, with midfacial prognathism |
|
Dentition |
Reduced, but large jaw size, creating retromolar gap |
|
Occipital Region |
Enlarged occipital region, occipital bun |
|
Other Unique Cranial Features |
Large infraorbital foramina |
|
Postcranial Features |
Short and stocky body, increased musculature, barrel-shaped chest |
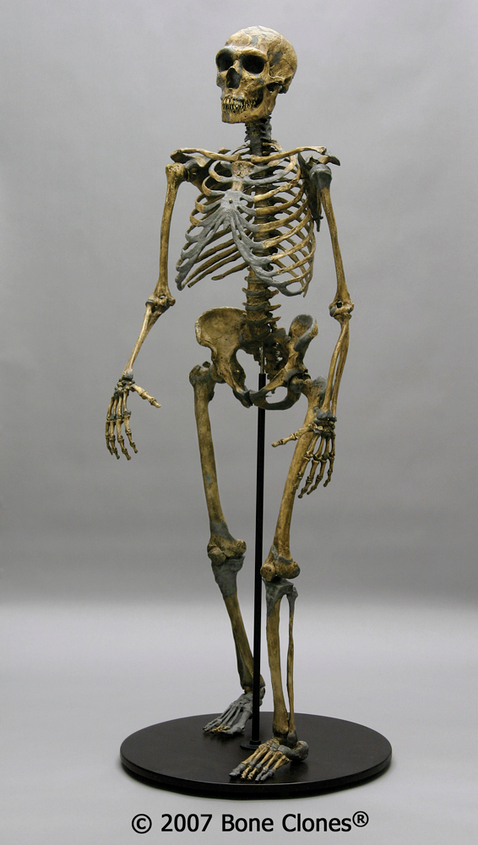
A classic example of a Neanderthal with all of the characteristics mentioned above is the nearly complete La Ferrassie 1 Neanderthal, from France. This is a male skeleton, with a brain size of around 1640cc, an extremely large nose and infraorbital foramina, brow ridges that are marked in size, and an overall robust skeleton (Figure 11.8).
Neanderthal Culture: Tool Making and Use
One key Neanderthal adaptation was their cultural innovations, which are an important way that hominins adapt to their environment. As you recall, Homo erectus‘s tools, Acheulean handaxes, represented an increase in complexity over Oldowan tools, allowing more efficient removal of meat and possibly calculated scavenging. In contrast, Neanderthal tools mark a significant innovation in tool-making technique and use with Mousterian tools (named after the Le Moustier site in southwest France). These tools were significantly smaller, thinner, and lighter than Acheulean handaxes and formed a true toolkit. The materials used for Mousterian tools were of higher quality, which allowed for both more precise toolmaking and tool reworking when the tools broke or dulled after frequent reuse. The use of higher-quality materials is also indicative of required forethought and planning to acquire them for tool manufacture. It is noteworthy that the Neanderthals, unlike Homo erectus, saved and reused their tools, rather than making new ones each time a tool was needed.
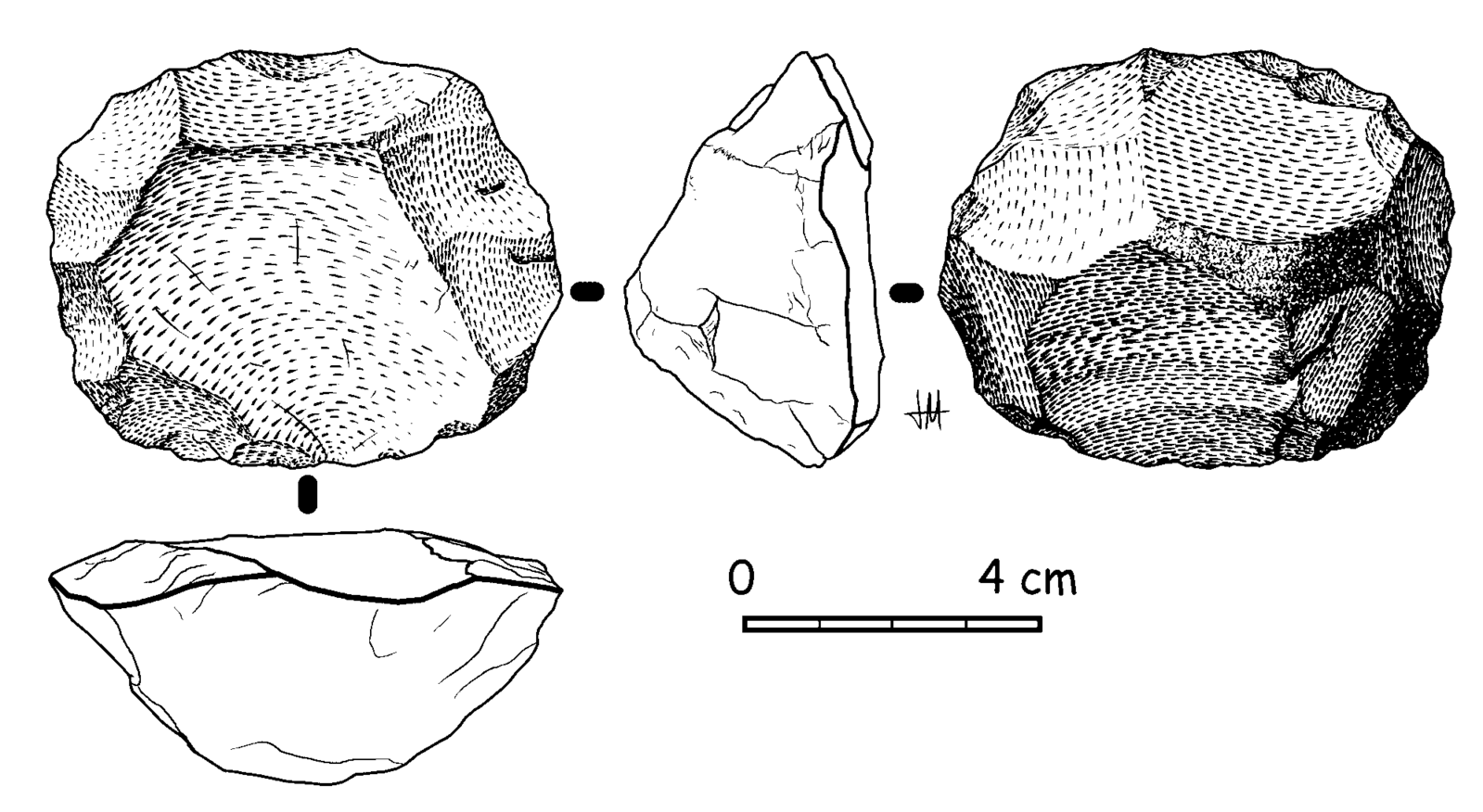
Mousterian tools are constructed in a very unique manner, utilizing the Levallois technique (Figure 11.9), named after the first finds of tools made with this technique, which were discovered in the Levallois-Perret suburb of Paris, France. The Levallois technique is a multistep process that requires preparing the core, or raw material, in a specific way that will yield flakes that are roughly uniform in dimension. The flakes are then turned into individual tools. The preparation of the core is akin to peeling a potato or carrot with a vegetable peeler—when peeling vegetables, you want to remove the skin in long, regular strokes, so that you are taking off the same amount of the vegetable all the way around. In the same way, the Levallois technique requires removing all edges of the cortex, or outside surface of the raw material, in a circle before removing the lid. The flakes, which will eventually be turned into the individual tools, can then be removed from the core. The potential yield of tools from one core would be many, as seen in Figure 11.10, compared to all previous tool-making processes, in which one core yielded a single tool. This manufacturing process might be considered the ultimate zero-waste tool-making technique (Delpiano et al. 2018).

Neanderthal tools were used for a variety of purposes, including cutting, butchering, woodworking or antler working, and hide working. Additionally, because the Mousterian tools were lighter than previous stone tools, Neanderthals could haft, or attach the tool onto a handle, as the stone would not have been too heavy (Degano et al. 2019). Neanderthals attached small stone blades onto short wood or antler handles to make knives or other small weapons, as well as attached larger blades onto longer shafts to make spears. New research examining tar-covered stones and black lumps at several Neanderthal sites in Europe suggests that Neanderthals may have been making tar by distilling it from birch tree bark, which could have been used to glue the stone tool onto its handle. If Neanderthals were, in fact, manufacturing tar to act as glue, this would predate modern humans in Africa using tree resin or similar adhesives by nearly 100,000 years.
Evidence shows that raw materials used by Neanderthals came from distances as far away as 100 km. This could indicate a variety of things regarding Neanderthal behavior, including a limited trade network with other Neanderthal groups or simply a large area scoured by Neanderthals when collecting raw materials. While research on specific applications continues, it should be clear from this brief discussion that Neanderthal tool manufacturing was much more complex than previous tool-making efforts, requiring technical expertise, patience, and skills beyond toolmaking to carry out.
Neanderthal Culture: Hunting and Diet
With their more sophisticated suite of tools and robust muscular bodies, Neanderthals were better armed for hunting than previous hominins. The animal remains in Neanderthal sites show that, unlike earlier Archaic Homo sapiens, Neanderthals were very effective hunters who were able to kill their own prey, rather than relying on scavenging. Though more refined than the tools of earlier hominins, the Neanderthal spear was not the kind of weapon that would have been thrown; rather, it would have been used in a jabbing fashion (Churchill 1998; Kortlandt 2002). This may have required Neanderthals to hunt in groups rather than individually and made it necessary to approach their prey quite closely (Gaudzinski-Windheuser et al. 2018). Remember, the animals living with Neanderthals were very large-bodied due to their adaptations to cold weather; this would have included species of deer, horses, and bovids (relatives of the cow).
Isotopes from Neanderthal bones show that meat was a significant component of their diet, similar to that seen in carnivores like wolves (Bocherens et al. 1999; Jaouen et al. 2019; Richards et al. 2000). In addition to large prey, their diet included ibex, seals, rabbits, and pigeons. Though red meat was a critical component of the Neanderthal diet, evidence shows that at times they also ate limpets, mussels, and pine nuts. Tartar examined from Neanderthal teeth in Iraq and Belgium reveal that they also ate plant material including wheat, barley, date palms, and tubers, first cooking them to make them palatable (Henry et al. 2010). While Neanderthals’ diet varied according to the specific environment in which they lived, meat comprised up to 80% of their diet (Wiẞin et al. 2015).
Neanderthal Culture: Caring for the Injured and Sick
While the close-range style of hunting used by Neanderthals was effective, it also had some major consequences. Many Neanderthal skeletons have been found with significant injuries, which could have caused paralysis or severely limited their mobility. Many of the injuries are to the head, neck, or upper body. Thomas Berger and Erik Trinkaus (1995) conducted a statistical comparative analysis of Neanderthal injuries compared to those recorded in modern-day workers’ compensation reports and found that the closest match was between Neanderthal injuries and those of rodeo workers. Rodeo professionals have a high rate of head and neck injuries that are similar to the Neanderthals’ injuries. What do Neanderthals and rodeo workers have in common? They were both getting very close to large, strong animals, and at times their encounters went awry.
The extensive injuries sustained by Neanderthals are evident in many fossil remains. Shanidar 1 (Figure 11.11), an adult male found at the Shanidar site in northern Iraq and dating to 45,000 ya, has a lifetime of injuries recorded in his bones (Stewart 1977). Shanidar 1 sustained—and healed from—an injury to the face that would have likely caused blindness. His lower right arm was missing and the right humerus shows severe atrophy, likely due to disuse. This pattern has been interpreted to indicate a substantial injury that required or otherwise resulted in amputation or wasting away of the lower arm. Additionally, Shanidar 1 suffered from bony growths in the inner ear that would have significantly impaired his hearing and severe arthritis in the feet. He also exhibited extensive anterior tooth wear, matching the pattern of wear found among modern populations who use their teeth as a tool. Rather than an anomaly, the type of injuries evident in Shanidar 1 are similar to those found in many other Neanderthal fossils, revealing injuries likely sustained from hunting large mammals as well as demonstrating a long life of physical activity.
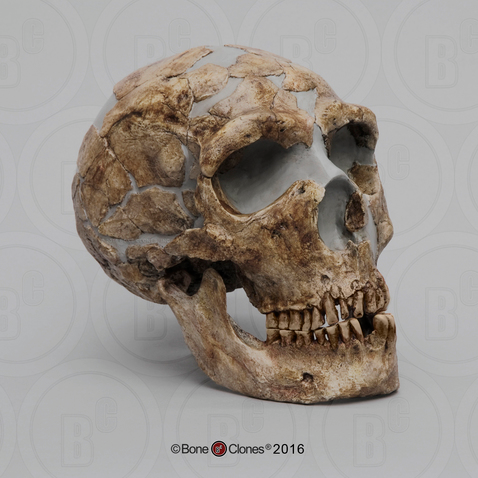

The pattern of injuries is as significant as the fact that Shanidar 1 and other injured Neanderthals show evidence of having survived their severe injuries. One of the earliest-known Neanderthal discoveries—the one on whom misinformed analysis shaped the stereotype of the species for nearly a century—is the La Chapelle-aux-Saints Neanderthal (Trinkaus 1985). The La Chapelle Neanderthal had a damaged eye orbit that likely caused blindness and suffered arthritis of the spine (Dawson and Trinkaus 1997). He had also lost most of his teeth, many of which he had lived without for so long that the mandibular and maxillary bones were partially reabsorbed due to lack of use. The La Chapelle Neanderthal was also thought to be at least in his mid-forties at death, an old age for the rough life of the Late Pleistocene—giving rise to his nickname, “the Old Man.” To have survived so long with so many injuries that obviously precluded successful large game hunting, he must have been taken care of by others. Such caretaking behavior is also evident in the survival of other seriously injured Neanderthals, such as Shanidar 1. Long thought to be a hallmark modern human characteristic, taking care of the injured and elderly, for example preparing or pre-chewing food for those without teeth, indicates strong social ties among Neanderthals.
Neanderthal Culture: Ritual Life
Such care practices may have been expressed upon death as well. Nearly complete Neanderthal skeletons are not uncommon in the fossil record, and most are well preserved within apparently deliberate burials that involve deep graves and bodies found in specific, often fetal or flexed positions (Harrold 1980). Discoveries of pollen in a grave at the Shanidar site in the 1960s led scientists to think that perhaps Neanderthals had placed flowering plants in the grave, an indication of ritual ceremony or spirituality so common in modern humans. But more recent investigations have raised some doubt about this conclusion (Pomeroy et al. 2020). The pollen may have been brought in by burrowing rodents. Claims of grave goods or other ornamentation in burials are similarly debated, although possible.
Some tantalizing evidence for symbolism, and debatably, ritual, is the frequent occurrence of natural pigments, such as ochre (red) and manganese dioxide (black) in Neanderthal sites that could have been used for art. However, the actual uses of pigments are unclear, as there is very little evidence of art or paintings in Mousterian sites. One exception may be the recent discovery in Spain of a perforated shell that appears to be painted with an orange pigment, which may be evidence of Neanderthal art and jewelry. However, many pigments also have properties that make them good emulsifiers in adhesive (like for attaching a stone tool to a wooden handle) or useful in tanning hides. So the presence of pigment may or may not be associated with symbolic thought; however, it definitely does show a technological sophistication beyond that of earlier Archaic hominins.
The Lasting Gift of Neanderthals: Tantalizing New Directions for Research
Examining the more recent time period in which Neanderthals lived and the extensive excavations completed across Europe allows for a much more complete archaeological record from this time period. Additionally, the increased cultural complexity such as complex tools and ritual behaviors expressed by Neanderthals left a more detailed record than previous hominins. Intentional burials enhanced preservation of the dead and potentially associated ritual behaviors. Such evidence allows for a more complete and nuanced picture of this species.

Additional analyses are possible on many Neanderthal finds, due to increased preservation of bone, the amount of specimens that have been uncovered, and the recency in which Neanderthals lived. We should be cautious, however, to consider the potential bias of many Neanderthal sites. Overwhelmingly, Neanderthal skeletons are found complete, with injuries or evidence of disease in caves. Does this mean all Neanderthals lived a tough, disease-wrought life? Probably not. It does, however, indicate that the sick were cared for by others, and that they lived in environments that preserved their bodies incredibly well. These additional studies include the examination of dental calculus and even DNA analysis. While limited, samples of Neanderthal DNA have been successfully extracted and analyzed. Research thus far has identified specific genetic markers that show some Neanderthals were light-skinned and probably red-haired with light eyes. Genetic analyses, different from the typical hominin reconstruction done with earlier species, allow scientists to further investigate soft tissue markers of Neanderthals and other more recent hominin species. These studies offer striking conclusions regarding Neanderthal traits, their physical appearance, and their culture, as reflected in these artists’ reconstructions (Figure 11.12).

Dr. Svante Pääbo (Figure 11.13), of the Max Planck Institute for Evolutionary Anthropology, has been at the forefront of much of this new research, largely in the form of genomic studies (The Nobel Prize 2022). Awarded the Nobel Prize for Physiology or Medicine in 2022, Pääbo is known primarily for his work with ancient DNA. He has successfully sequenced mitochondrial DNA (mtDNA) as well as the entire Neanderthal genome from nuclear DNA. His genomic work has led to the realization that Denisovans are genetically distinct from Neanderthals, as well as the recent identification of a Neanderthal father and teenage daughter, which he discovered by looking for unique DNA markers in the fossil record. Additionally, Pääbo’s genomic work has provided researchers with additional lines of evidence regarding the connections between hominin fossils (such as Neanderthals) and modern people, their time of divergence, and current genetic overlap. The work of Pääbo has even formalized a new field of study within anthropology—paleogenomics. To stay up to date with Dr. Svante Pääbo’s work, be sure to follow his lab’s website.
Neanderthal Culture: Communicating through Speech
To successfully live in groups and to foster cultural innovations, Neanderthals would have required at least a basic form of communication in order to function, possibly using a speech-based communication system. The challenge with this line of research is that speech, of course, is not preserved, so indirect evidence must be used to support this conclusion. It is thought that Neanderthals would have possessed some basic speech, as evidenced from a variety of sources, including throat anatomy and genetic evidence (Lieberman 1971). There is only one bone in the human body that could demonstrate if a hominin was able to speak, or produce clear vocalizations like modern humans, and that is the hyoid, a U-shaped bone that is found in the throat and is associated with the ability to precisely control the vocal cords. Very few hyoid bones have been found in the archaeological record; however, a few have been uncovered in Neanderthal burials. The shape of the Neanderthal hyoid is nearly identical to that of modern humans, pointing to the likelihood that they had the same vocal capabilities as modern humans. In addition, geneticists have uncovered a mutation present in both modern humans and Neanderthals—the FOXP2 gene—that is possibly linked to the ability to speak. However, other scientists argue that we cannot make sweeping conclusions that the FOXP2 gene accounts for speech due to small sample size. Finally, scientists have also pointed to the increasingly complex cultural behavior of Neanderthals as a sign that symbolic communication, likely through speech, would have been the only way to pass down the skills needed to make, for example, a Levallois blade or to position a body for intentional burial.
Neanderthal Intelligence
One of the enduring questions about Neanderthals centers on their intelligence, specifically in comparison to modern humans. Brain volume indicates that Neanderthals certainly had a large brain, but it continues to be debated if Neanderthals were of equal intelligence to modern humans. Remember, creatures with larger body sizes tend to have larger brains; however, scaling of the brain is not always associated with greater intelligence (Alex 2018). Brain volume (cranial capacity), cultural complexity, tool use, and compassion toward their kind all point to an increase in intellect among Neanderthals when compared to previous hominins.
Yet, new research is suggesting additional differences between Neanderthal brains and our own. For example, Euluned Pearce and colleagues (2013), from the University of Oxford, noted the frontal lobes of Neanderthals and modern humans are almost identical. However, Neanderthals had a larger visual cortex—the portion of the brain involved in processing visual information. This would have left Neanderthals with less brain tissue for other functions, including those that would have aided them in dealing with large social groupings, one of the differences that has been suggested to exist between Neanderthals and modern humans. Other differences were found when geneticist John Blangero, from the Texas Biomedical Research Institute, compared data from the Neanderthal genome against data from modern study participants. Blangero and his colleagues (Blangero et al. 2014) discovered that some Neanderthal brain components were very different, and smaller, than those in the modern sample. Differences were found in areas associated with the processing of information and controlling emotion and motivation, as well as overall brain connectivity. In short, as Blangero stated, “Neanderthals were certainly cognitively adept,” although their specific abilities may have differed from modern humans’ in key areas (qtd. in Wong 2015). This point has been echoed in other recent genetic studies comparing Neanderthal and anatomically modern human brains (el-Showk 2019).
Finally, scientists are fairly certain that Neanderthal brain development after birth was not the same as that of modern humans. After birth, anatomically modern Homo sapiens babies go through a critical period of brain expansion and cognitive development. It appears that Neanderthal babies’ brains and bodies did not follow the same developmental pattern (Smith et al. 2010; Zollikofer and Ponce de León 2013). Modern humans enjoy an extended period of childhood, which allows children to engage in imaginative play and develop creativity that fosters cognitive skills. Neanderthals had a more limited childhood, with less development of the creative mind that may have affected their species’ success (Nowell 2016).
The exact nature of Neanderthal intelligence remains under investigation, however. Some studies disagree with the idea that Neanderthal intelligence had limitations compared to our own, noting the extensive evidence of Neanderthals having limb asymmetry. Their tools also have wear marks indicating that they were hand-dominant. This is further supported by marks on Neanderthal teeth that demonstrate hand dominance. The Neanderthal “stuff-and-cut method” of eating, noted by David Frayer and colleagues (Frayer et al. 2012), would have seen Neanderthals hold a piece of meat in their teeth, while pulling it taut with one hand, and then using the other hand, their dominant one, to cut the meat off of the larger slab being held in their teeth. When looking at 17 Neanderthals and their tooth wear, only two do not show markings associated with a right-hand dominant individual eating in this manner. Further, it has been established that favoring the right hand is a key marker between modern humans and chimpanzees, and that handedness also relates to language development, in the form of bilateral brain development. That Neanderthals likely were hand-dominant suggests they had an indicator of bilateral brain development and a precondition for human speech.
The Middle Stone Age: Neanderthal Contemporaries in Africa
While Neanderthals made their home on and adapted to the European and Asian continents, evidence of fossil humans in Africa show they were also adapting to their local environments. These populations in Africa exhibit many more similarities to modern humans than Neanderthals, as well as overall evolutionary success. While the African fossil sample size is smaller and more fragmentary than the number of Neanderthal specimens across Europe and Asia, the African sample is interesting in that it represents a longer time period and larger geographical area. This group of fossils—often represented by the name “Middle Stone Age,” or MSA—dates to between 300,000 and 30,000 years ago across the entire continent of Africa. As with Archaic Homo sapiens, there is much variability seen in this African set of fossils. There are also a few key consistent elements: none of them exhibit Neanderthal skeletal features; instead, they demonstrate features that are increasingly consistent with anatomically modern Homo sapiens.
Similarities to Neanderthals and MSA contemporaries in Africa are seen, however, in their behavioral adaptations, including stone tools and other cultural elements. The tools associated with the specimens living in Africa during this time period are, like their physical features, varied. In some parts of Africa, namely Northern Africa, stone tools from this time so closely resemble Neanderthal tools that they are classified as Mousterian. In sub-Saharan Africa, the stone tools associated with these specimens are labeled as MSA. Some scholars argue that these could also be a type of Mousterian tools, but they are still typically subdivided based on geographical location.
Recall that Mousterian tools were much more advanced than their Acheulean predecessors in terms of how the stone tools were manufactured, the quality of the stones used, and the ultimate use of the tools that were made. In addition, recent evidence suggests that MSA tools may also have been heat treated—to improve the quality of the stone tool produced (Stolarczyk and Schmidt 2018). Evidence for heat treating is seen not only through advanced analysis of the tool itself but also through the residue of fires from this time period. Fire residues show a shift over time from small, short fires fueled by grasses (probably intended for cooking) to larger, more intensive fires that required the exploitation of dry wood, exactly the type of fire that would have been needed for heat treating stone tools (Esteban et al. 2018).
Other cultural elements seen with MSA specimens include the use of marine (sea-based) resources for their diet (Parkington 2003), manufacture of bone tools, use of adhesive and compound tools (e.g., hafted tools), shell bead production, engraving, use of pigments (such as ochre), and other more advanced tool-making technology (e.g., microlithics). While many of these cultural elements are also seen to a limited extent among Neanderthals, developments at MSA sites appear more complex. This MSA cultural expansion may have been a response to climate change or an increased use of language, complex communication, and/or symbolic thought. Others have suggested that the MSA cultural expansion was due to the increase of marine resources in their diet, which included more fatty acids that may have aided their cognitive development. Still others have suggested that the increased cultural complexity was due to increased interaction among groups, which spurred competition to innovate. Recent studies suggest that perhaps the best explanation for the marked cultural complexity of MSA cultural artifacts is best explained by the simple fact that they lived in diverse habitats (Kandel et al. 2015). This would have necessitated a unique set of cultural adaptations for each habitat type (for example, specialized marine tools would have been needed along coastal sites but not at inland locations). Simply put, the most useful adaptation of MSA was their flexibility of behavior and adaptability to their local environment. As noted previously in this chapter, flexibility of behavior and physical traits, rather than specialization, seems to be a feature that was favored in hominin evolution at this time.
Where Did They Go? The End of Neanderthals
While MSA specimens were increasingly successful and ultimately transitioned into modern Homo sapiens, Neanderthals disappeared from the fossil record by around 40,000 years ago. What happened to them? We know, based on genetics, that modern humans come largely from the modern people who occupied Africa around 300,000 to 100,000 years ago, at the same time that Neanderthals were living in northern Europe and Asia. As you will learn in Chapter 12, modern humans expanded out of Africa around 150,000 years ago, rapidly entering areas of Europe and Asia inhabited by Neanderthals and other Archaic hominins. Despite intense interest and speculation in fictional works about possible interactions between these two groups, there is very little direct evidence of either peaceful coexistence or aggressive encounters. It is clear, though, that these two closely related hominins shared Europe for thousands of years, and recent DNA evidence suggests that they occasionally interbred (Fu et al. 2015). Geneticists have found traces of Neanderthal DNA (as much as 1% to 4%) in modern humans of European and Asian descent not present in modern humans from sub-Saharan Africa. This is indicative of limited regional interbreeding with Neanderthals.
While some interbreeding likely occurred, as a whole, Neanderthals did not survive. What is the cause for their extinction? This question has fascinated many researchers and several possibilities have been suggested, including:
- At the time that Neanderthals were disappearing from the fossil record, the climate went through both cooling and warming periods—each of which posed challenges for Neanderthal survival (Defleur and Desclaux 2019; Staubwasser et al. 2018). It has been argued that as temperatures warmed, large-bodied animals, well adapted to cold weather, moved farther north to find colder environments or faced extinction. A shifting resource base could have been problematic for continued Neanderthal existence, especially as additional humans, in the form of modern Homo sapiens, began to appear in Europe and compete for a smaller pool of available resources.
- It has been suggested that the eruption of a European volcano 40,000 years ago could have put a strain on available plant resources (Golovanova et al. 2010). The eruption would have greatly affected local microclimates, reducing the overall temperature enough to alter the growing season.
- Possible differences in cognitive development may have limited Neanderthals in terms of their creative problem solving. As much as they were biologically specialized for their environment, the nature of their intelligence might not have offered them the creative problem-solving skills to innovate ways to adapt their culture when faced with a changing environment (Pearce et al. 2013).
- CRISPR gene-editing technology has been used in studies to evaluate potential differences between human and Neanderthal brains, based on differences in the genetic code. Potential differences include a Neanderthal propensity for mutations related to brain development that could account for more rapid brain development, maturation, synapse misfires, and less-orderly neural processes (Mora-Bermúdez et al. 2022; Trujillo et al. 2021). Fundamental differences in brain function at the cellular level may account for the differential survival rates of Neanderthal and modern human populations.
- There is evidence that suggests reproduction may have posed challenges for Neanderthals. Childbirth was thought to have been at least as difficult for female Neanderthals as anatomically modern Homo sapiens (Weaver and Hublin 2009). Female Neanderthals may have become sexually mature at an older age, even older than modern humans. This delayed maturation could have kept the Neanderthal population size small. A recent study has further suggested that male Neanderthals might have had a genetic marker on the Y chromosome that could have caused incompatibility between the fetus and mother during gestation; this would have had severe consequences for birth rate and survival (Mendez et al. 2016). Even a small but continuous decrease in fertility would have been enough to result in the extinction of Neanderthals (Degioanni et al. 2019).
- As mentioned above, the end of Neanderthal existence overlaps with modern human expansion into northern Europe and Asia. There is no conclusive direct evidence to indicate that Neanderthals and modern humans lived peacefully side by side, nor that they engaged in warfare, but by studying modern societies and the tendencies of modern humans, it has been suggested that modern humans may not have warmly embraced their close but slightly odd-looking cousins when they first encountered them (Churchill et al. 2009). Nevertheless, direct competition with modern humans for the same resources may have contributed to the Neanderthals’ decline (Gilpin et al. 2016); it may also have exposed them to new diseases, brought by modern humans (Houldcroft and Underdown 2016), which further decimated their population. Estimates of energy expenditures suggest Neanderthals had slightly higher caloric needs than modern humans (Venner 2018). When competing for similar resources, the slightly greater efficiency of modern humans might have helped them experience greater success in the face of competition—at a cost to Neanderthals.
As Neanderthal populations were fairly small to begin with (estimated between 5,000 and 70,000 individuals; Bocquet-Appel and Degioanni 2013), one or a combination of these factors could have easily led to their demise. As more research is conducted, we will likely get a better picture of exactly what led to Neanderthal extinction.
Denisovans

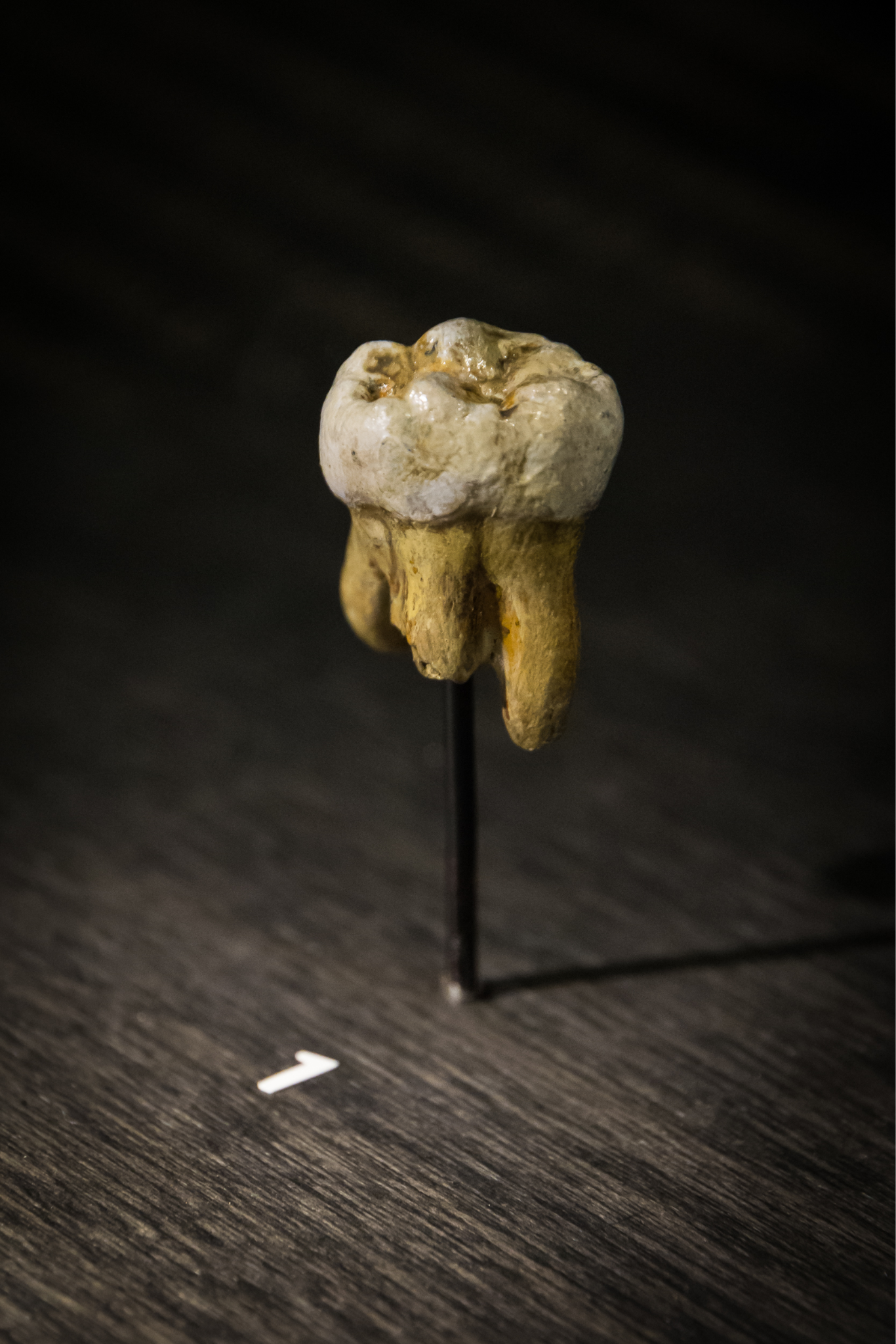
While Neanderthals represent one regionally adapted branch of the Archaic Homo sapiens family tree, recent discoveries in Siberia and the Tibetan Plateau surprised paleoanthropologists by revealing yet another population that was contemporary with Archaic Homo sapiens, Neanderthals, and modern Homo sapiens. The genetic analysis of a child’s finger bone (Figure 11.14) and an adult upper third molar (Figure 11.15) from Denisova Cave in the Altai Mountains in Siberia by a team including Svante Pääbo discovered that the mitochondrial and nuclear DNA sequences reflected distinct genetic differences from all known Archaic populations. Dubbed “Denisovans” after the cave in which the bones were found, this population is more closely related to Neanderthals than modern humans, suggesting the two groups shared an ancestor who split from modern humans first, then the Neanderthal-Denisovan line diverged more recently (Reich et al. 2010).
Denisovans share up to 5% of their DNA with modern Melanesians, aboriginal Australians, and Polynesians, and 0.2% of their DNA with other modern Asian populations and Native Americans. Additional studies have suggested one (Vernot et al. 2018) or two (Browning et al. 2018) separate points of time when interbreeding occurred between modern humans and Denisovans.
Genetic analysis reveals that Denisovans (potentially three distinct populations) had adaptations for life at high altitudes that prevented them from developing altitude sickness and hypoxia in extreme environments such as Tibet, where the average annual temperature is close to 0℃ and the altitude is more than a kilometer (about 4,000 feet) above sea level. Through protein analysis of a jawbone, one study (Chen et al. 2019) has placed Denisovans in Tibet as early as 160,000 years ago. Genetic evidence of interbreeding has linked modern Tibetan populations with Denisovans 30,000 to 40,000 years ago, which implies that the unique high-altitude adaptations seen in modern Tibetans may have originated with Denisovans (Huerta-Sanchez et al. 2014).
Other research suggests tantalizing new directions regarding Denisovans. Stone tools similar to those found in Siberia have been uncovered in the Tibetan plateau suggesting a connection between the Denisovan populations in those two areas (Zhang et al. 2018). The molar of a young girl, possibly Denisovan, has been found in Laos and shows strong similarities to specimens from China (Demeter et al. 2022). And DNA sequencing from discoveries in the Denisova Cave have yielded a genome that has been interpreted as the first-generation offspring of a Denisovan father and Neanderthal mother (Slon et al. 2018). While this research is not yet conclusive and is still being interpreted, exciting new possibilities are being revealed. To stay up-to-date with new discoveries, consider following organizations such as the Smithsonian’s Human Origins Program on social media.
How Do These Fit In? Homo naledi and Homo floresiensis
Recently, some fossils have been unearthed that have challenged our understanding of the hominin lineage. The fossils of Homo naledi and Homo floresiensis are significant for several reasons but are mostly known for how they don’t fit the previously held patterns of hominin evolution. While we examine information about these species, we ask you to consider the evidence presented in this chapter and others to draw your own conclusions regarding the significance and placement of these two unusual fossil species in the hominin lineage.
Homo naledi
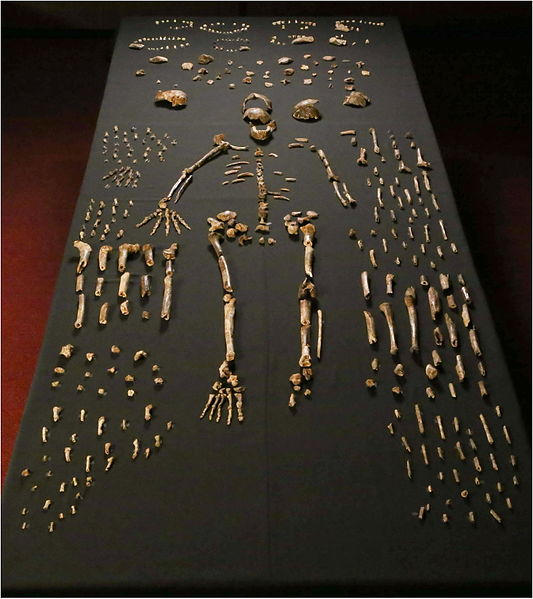
In 2013 recreational spelunkers uncovered a collection of bones deep in a cave network in Johannesburg, South Africa. The cave system, known as Rising Star, had been well documented by other cavers; however, it appears few people had ever gone as far into the cave as these spelunkers did. Lee Berger, paleoanthropologist at University of Witwatersrand, in Johannesburg, immediately put out a call for what he termed “underground astronauts” to begin recovery and excavation of the fossil materials. Unlike other excavations, Berger and most other paleoanthropologists would not be able to access the elusive site, as it was incredibly difficult to reach, and at some points there was only eight inches of space through which to navigate. The underground astronauts, all petite, slender female anthropologists, were the only ones who were able to access this remarkable site. Armed with small excavation tools and a video camera, which streamed the footage up to the rest of the team at the surface, the team worked together and uncovered a total of 1,550 bones, representing at least 15 individuals, as seen in Figure 11.16. Later, an additional 131 bones, including an almost-complete cranium, were found in a nearby chamber of the cave, representing three more individuals (Figure 11.17). Berger called in a team of specialists to participate in what was dubbed “Paleoanthropology Summer Camp.” Each researcher specialized in a different portion of the hominin skeleton. With various specialists working simultaneously, more rapid analysis was possible of Homo naledi than most fossil discoveries.
While access to the site, approximately 80 m from any known cave entrance or opening, was treacherous for researchers, it must have been difficult for Homo naledi as well. The route included moving through a portion that is just 25 cm wide at some points, known as “Superman’s Crawl.” The only way to get through this section is by crawling on your stomach with one arm by your side and the other raised above your head. Past Superman’s Crawl, a jagged wall known as the Dragon’s Back would have been very difficult to traverse. Below that, a narrow vertical chute would have eventually led down to the area where the fossils were discovered. While geology changes over time and the cave system likely has undergone its fair share, it is not likely that these features arose after Homo naledi lived (Dirks et al. 2017). This has made scientists curious as to how the bones ended up in the bottom of the cave system in the first place. It has been suggested that Homo naledi deposited the bones there, one way or another. If Homo naledi did deposit the bones, either through random disposal or intentional burial, this raises questions regarding their symbolic behavior and other cultural traits, including the use of fire, to access a very dark cave system. Another competing idea is that a few individuals may have entered the cave system to escape a predator and then got stuck. To account for the sheer number of fossils, this would have had to happen multiple times.
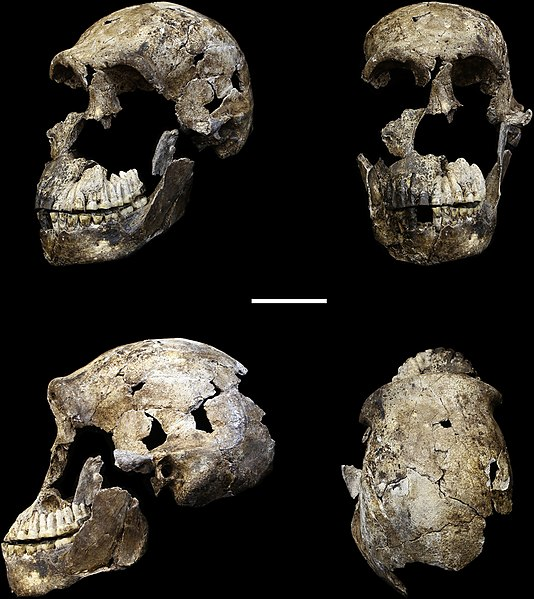
The features of Homo naledi are well-documented due to the fairly large sample, which represents individuals of all sexes and a wide range of ages. The skull shape and features are very much like other members of the genus Homo—including a sagittal keel and large brow, like Homo erectus, and a well-developed frontal lobe, similar to modern humans—yet the brain size is significantly smaller than its counterparts, at approximately 500 cc (560 cc for males and 465 cc for females). The teeth also exhibit features of later members of the genus Homo, such as Neanderthals, including a reduction in overall tooth size. Homo naledi also had unique shoulder anatomy and curved fingers, indicating similarities to tree-dwelling primates, which is very different from any other hominin yet found. Perhaps the greatest shock of all is that Homo naledi has been dated to 335,000 to 236,000 years ago, placing it as a contemporary to modern Homo sapiens, despite its very primitive features. An additional specimen of a child, found in 2021, not only shares many of the unique features found in the adult specimen but will also add insight into the growth and development of individuals of this species (Brophy et al. 2021).
Homo floresiensis
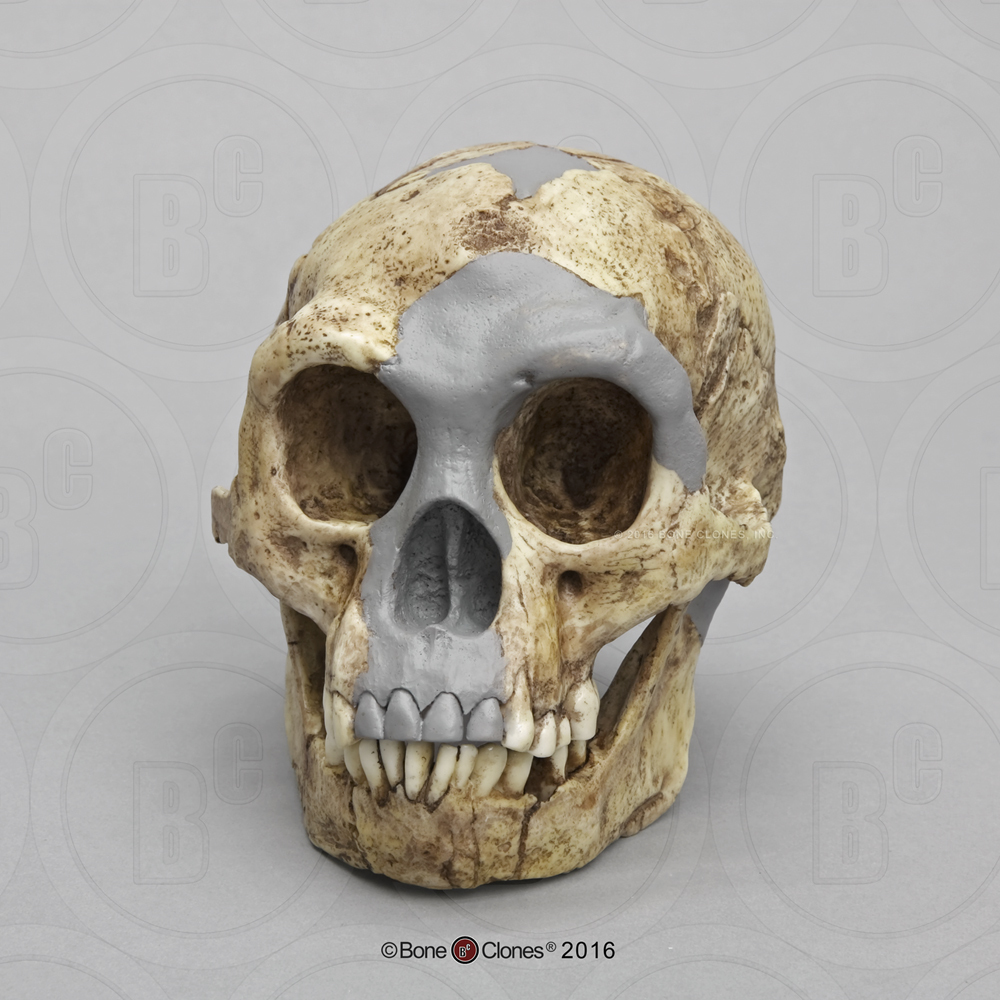
In a small cave called Liang Bua, on the island of Flores, in Indonesia, a small collection of fossils were discovered beginning in 2003 (Figure 11.18). The fossil fragments represent as many as nine individuals, including a nearly complete female skeleton. The features of the skull are very similar to that of Homo erectus, including the presence of a sagittal keel, an arching brow ridges and nuchal torus, and the lack of a chin (Figure 11.19). Homo floresiensis, as the new species is called, had a brain size that was remarkably small at 400 cc, and recent genetic studies suggest a common ancestor with modern humans that predates Homo erectus.

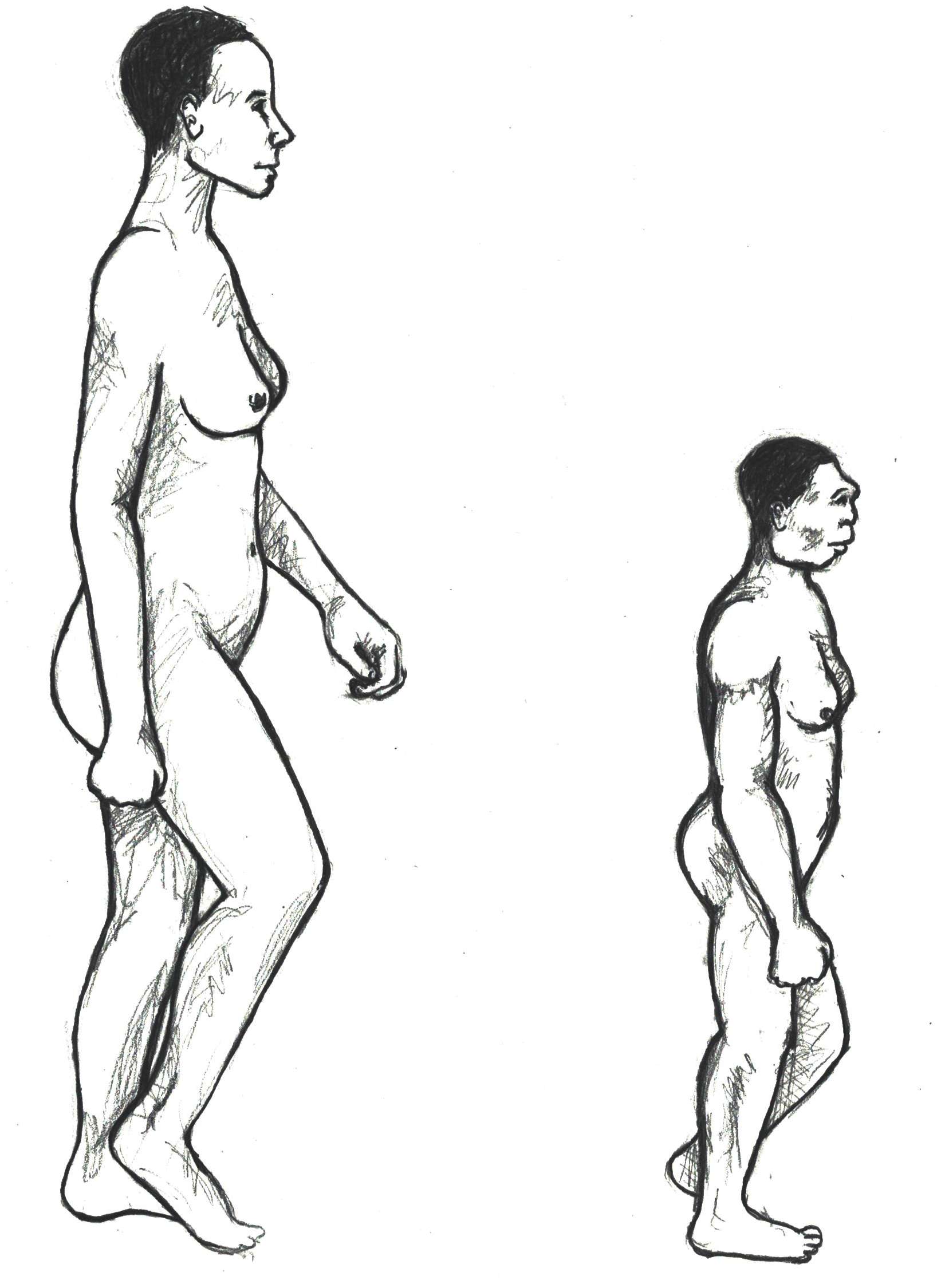
The complete female skeleton, who was an adult, was approximately a meter tall and would have weighed just under 30 kg, which is significantly shorter and just a few kilograms more than the average, modern, young elementary-aged child. A reconstructed comparison between an anatomically modern human and Homo floresiensis can be seen in Figure 11.20. The small size of the fossil has earned the species the nickname “the Hobbit.” Many questions have been asked about the stature of this species, as all of the specimens found also show evidence of diminutive stature and small brain size. Some explanations include pathology; however, this seems unlikely as all fossils found thus far demonstrate the same pattern. Another possible explanation lies in a biological phenomena seen in other animal species also found on the island, which date to a similar time period. This phenomenon, called insular dwarfing, is due to limited food resources on an island, which can create a selective pressure for large-bodied species to be selected for smaller size, as an island would not have been able to support their larger-bodied cousins for a long period of time. This phenomenon is the cause of other unique species known to have lived on the island at the same time, including the miniature stegodon, a dwarf elephant species.
There is ongoing research and debate regarding Homo floresiensis’ dates of existence, with some researchers concluding that they lived on Flores until perhaps as recently as 17,000 years ago, although they are more often dated to 100,000 to 60,000 years ago. Stone tools from that time period uncovered at the site are similar to other hominin stone tools found on the island of Flores. Homo floresiensis would have hunted a wide range of animals, including the miniature stegodon, giant rats, and other large rodents. Other animals on the island that could have threatened them include the giant komodo dragon. An interesting note about this island chain is that ancestors of Homo floresiensis would have had to traverse the open ocean in order to get there, as the nearest island is almost 10 km away, and there is little evidence to support that a land bridge connecting mainland Asia or Australia to the island would have been present. This separation from the mainland would also have limited the number of other animals, including predators and human species, that would have had access to the island. Anatomically modern Homo sapiens arrived on the island around 30,000 years ago and, if some researchers’ later dates for Homo floresiensis are correct, both species may have lived on Flores at the same time. The modern population living on the island of Flores today believes that their ancestors came from the Liang Bua cave; however, recent genetic studies have determined they are not related to Homo floresiensis (Tucci et al. 2018).
Conclusion
Research presented in the chapter contributes to why scientists have taken to nicknaming this time period “the muddle in the middle.” We know that the Middle Pleistocene picks up from Homo erectus and ends with the appearance of anatomically modern Homo sapiens. While the start and the end are clear, it’s the middle that is messy. As more research is conducted and more data is collected, rather than clarifying our understanding of the hominin lineage during this time period, it only inspires more questions, particularly about the relationships between hominins during this time period, including the oft-misunderstood Neanderthal. Research is painting a more detailed picture of Neanderthal intelligence and both biological and behavioral adaptations. At the same time, their relationship to other Middle Pleistocene hominins, including Denisovans, as well as modern humans, remains unclear.
Homo naledi and Homo floresiensis are clear outliers when compared to their contemporary hominin species. Each has surprised paleoanthropologists for both their archaic traits in relatively modern times and their unique combination of traits seen in archaic species and modern humans. While these finds have been exciting, they have also completely upended the assumed trajectory of the human lineage, causing scientists to re-examine assumptions about hominin evolution and what it means to be modern. Add this to the developments being made using ancient DNA, other new fossil discoveries, and other innovations in paleoanthropology, and you see that our understanding of Archaic Homo sapiens and others living during this time period is rapidly developing and changing. This is a true testament to the nature of science and the scientific method.
Clearly, hominins of the Middle Pleistocene are distinct from our species today. Yet, understanding the hominins that directly preceded our species and clarifying the evolutionary relationships between us is important to better understanding our own place in nature.
Hominin Species Summaries
|
Hominin |
Archaic Homo sapiens |
|---|---|
|
Dates |
600,000–200,000 years ago (although some regional variation) |
|
Region(s) |
Africa, Europe, and Asia |
|
Famous discoveries |
Broken Hill (Zambia), Atapuerca (Spain) |
|
Brain size |
1,200 cc average |
|
Dentition |
Slightly smaller teeth in back of mouth, larger front teeth |
|
Cranial features |
Emerging forehead, no chin, projecting occipital region |
|
Postcranial features |
Robust skeleton |
|
Culture |
Varied regionally, but some continue to use Acheulean handaxe, others adopt Mousterian tool culture |
|
Other |
Lots of regional variation in this species |
|
Species |
Homo naledi |
|---|---|
|
Dates |
335,000–236,000 years ago |
|
Region(s) |
South Africa |
|
Famous discoveries |
Rising Star Cave |
|
Brain size |
500 cc average |
|
Dentition |
Reduced tooth size |
|
Cranial features |
Sagittal keel, large brow, well-developed frontal region |
|
Postcranial features |
Suspensory shoulder |
|
Culture |
unknown |
|
Other |
N/A |
|
Hominin |
Neanderthals |
|---|---|
|
Dates |
150,000–40,000 years ago |
|
Region(s) |
Western Europe, Middle East, and Western Asia only |
|
Famous discoveries |
Shanidar (Iraq), La Chapelle-aux-Saints (France) |
|
Brain size |
1500 cc average |
|
Dentition |
Retromolar gap |
|
Cranial features |
Large brow ridge, midfacial prognathism, large infraorbital foramina, occipital bun |
|
Postcranial features |
Robust skeleton with short and stocky body, increased musculature, barrel chest |
|
Culture |
Mousterian tools often constructed using the Levallois technique |
|
Other |
N/A |
|
Species |
Homo floresiensis |
|---|---|
|
Dates |
100,000–60,000 years ago, perhaps as recently as 17,000 years ago |
|
Region(s) |
Liang Bua, island of Flores, Indonesia |
|
Famous discoveries |
“The Hobbit” |
|
Brain size |
400 cc average |
|
Dentition |
unknown |
|
Cranial features |
Sagittal keel, arching brow ridges, nuchal torus, no chin |
|
Postcranial features |
Very short stature (approximately 3.5 ft.) |
|
Culture |
Tools similar to other tools found on the island of Flores |
|
Other |
N/A |
|
Hominin |
Denisovans |
|---|---|
|
Dates |
100,000–30,000 years ago |
|
Region(s) |
Siberia |
|
Famous discoveries |
Child’s finger bone and adult molar |
|
Brain size |
unknown |
|
Dentition |
Large molars (from limited evidence) |
|
Cranial features |
unknown |
|
Postcranial features |
unknown |
|
Culture |
unknown |
|
Other |
Closely related to Neanderthals (genetically) |
Review Questions
- What physical and cultural features are unique to Archaic Homo sapiens? How are Archaic Homo sapiens different in both physical and cultural characteristics from Homo erectus?
- Describe the specific changes to the brain and skull first seen in Archaic Homo sapiens. Why does the shape of the skull change so dramatically from Homo erectus?
- What role did the shifting environment play in the adaptation of Archaic Homo sapiens, including Neanderthals? Discuss at least one physical feature and one cultural feature that would have assisted these groups in surviving the changing environment.
- What does the regional variation in Archaic Homo sapiens represent in terms of the broader story of our species’ evolution?
- Describe the issues raised by the discoveries of Homo naledi and Homo floresiensis in the understanding of the story of the evolution of Homo sapiens.
Key Terms
Allele: Each of two or more alternative forms of a gene that arise by mutation and are found at the same place on a chromosome.
Anthropocentrism: A way of thinking that assumes humans are the most important species and leads to interpreting the world always through a human lens. Species-centric science and thought.
Cortex: The outside, or rough outer covering, of a rock. Usually the cortex is removed during the process of stone tool creation.
Ethnocentric: Applying negative judgments to other cultures based on comparison to one’s own.
Exogenous DNA: DNA that originates from sources outside of the specimen you are trying to sequence.
Flexed position: Fetal position, in which the legs are drawn up to the middle of the body and the arms are drawn toward the body center. Intentional burials are often found in the flexed body position.
Foraminifera: Microscopic single-celled organisms with a shell that are common in all marine environments. The fossil record of foraminifera extends back well over 500 million years.
Glaciation: A glacial period, or time when a large portion of the world is covered by glaciers and ice sheets.
Globular: Round-shaped, like a globe.
Grave goods: Items included with a body at burial. Items may signify occupation or hobbies, social status, or level of importance in the community, or they may be items believed necessary for the afterlife.
Haft: A handle. Also used as a verb—to attach a handle to an item, such as a stone tool.
Infraorbital foramina: Small holes on the maxilla bone of the face that allows nerves and blood to reach the skin.
Insular dwarfing: A form of dwarfism that occurs when a limited geographic region, such as an island, causes a large-bodied animal to be selected for a smaller body size.
Interglacial: A warmer period between two glacial time periods.
Levallois technique: A distinctive technique of stone tool manufacturing used by Archaic Homo sapiens, including Neanderthals. The technique involves the preparation of a core and striking edges off in a regular fashion around the core. Then a series of similarly sized pieces can be removed, which can then be turned into different tools.
Midfacial prognathism: A forward projection of the nose or the middle facial region. Usually associated with Neanderthals.
Mousterian tools: The stone tool industry of Neanderthals and their contemporaries in Africa and Western Asia. Mousterian tools are known for a diverse set of flake tools, which is different from the large bifacial tools of the Acheulean industry.
Nasal aperture: The opening for the nose visible on a skull. Often pear- or heart-shaped.
Occipital bun: A prominent bulge or projection on the back of the skull, specifically the occipital bone. This is a feature present only on Neanderthal skulls.
Ochre: A natural clay pigment mixed with ferric oxide and clay and sand. Ranges in color from brown to red to orange.
Retracted face: A face that is flatter.
Retromolar gap: A space behind the last molar and the end of the jaw. This is a feature present only on Neanderthals. It also occurs through cultural modification in modern humans who have had their third molars, or wisdom teeth, removed.
About the Authors
 Amanda Wolcott Paskey, M.A.
Amanda Wolcott Paskey, M.A.
Cosumnes River College, paskeya@crc.losrios.edu
Amanda Wolcott Paskey is an anthropology professor at Cosumnes River College in Sacramento, California. She earned her B.A. and M.A. in anthropology from the University of California, Davis. Her speciality in anthropology is archaeology; however, she was trained in a holistic program and most of her teaching load is in biological anthropology. She is currently working on analyzing a post–gold rush era archaeological site, in Sacramento, with colleagues and students. This project has given her many opportunities to engage in sharing archaeology with a public audience, including local school children and Sacramentans interested in local history.
 AnnMarie Beasley Cisneros, M.A.
AnnMarie Beasley Cisneros, M.A.
American River College, beaslea@arc.losrios.edu
AnnMarie Beasley Cisneros is an anthropology professor at American River College in Sacramento, California. Trained as a four-field anthropologist, she earned her B.A. and M.A. in anthropology from California State University, Sacramento. She regularly teaches biological anthropology, among other courses, and is currently engaged in applied anthropology work in community development with historically underserved communities. She most recently has particularly enjoyed facilitating her students’ involvement in projects serving Sacramento’s Latino and immigrant Mexican populations.
For Further Exploration
Anne and Bernard Spitzer Hall of Human Origins—American Museum of Natural History.
“Dawn of Humanity,” PBS documentary, 2015
“DNA Clues to Our Inner Neanderthal,” TED Talk by Svante Pääbo, 2011.
“The Dirt” Podcast, Episode 30, “The Human Family Tree (Shrub? Crabgrass? Tumbleweed?), Part 3: Very Humany Indeed”.
Frank, Rebecca. 2021. “The Genus Homo.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Hobbits on Flores, Indonesia – Smithsonian Human Origins.
Lumping or Splitting in the Fossil Record – UC Berkeley Understanding Evolution.
Neandertals and More – Max Planck Institute for Evolutionary Anthropology.
Neanderthals: Body of Evidence – SAPIENS.
Perash, Rose L., and Kristen A. Broehl. 2021. “Hominin Review: Evolutionary Trends.” In Explorations: Lab and Activity Manual, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. Arlington, VA: American Anthropological Association.
Perkl, Bradley. “Brain, Language, Lithics.” In Explorations: Lab and Activity Manual, edited by. Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff. CC BY-NC. Arlington, VA: American Anthropological Association.
Shanidar 3 – Neanderthal Skeleton – Smithsonian Human Origins.
Species – Smithsonian Human Origins.
Smithsonian Human Origins Program Facebook page (@smithsonian.humanorigins).
Paleoartist Brings Human Evolution to Life – Elisabeth Daynés.
References
Adler, Daniel S., Timothy J. Prindiville, and Nicholas J. Conard. 2003. “Patterns of Spatial Organization and Land Use During the Eemian Interglacial in the Rhineland: New Data from Wallertheim, Germany.” Eurasian Prehistory 1(2): 25–78.
Alex, Bridget. 2018. “Neanderthal Brains: Bigger, Not Necessarily Better.” Discover, September 21, 2018. https://www.discovermagazine.com/planet-earth/neanderthal-brains-bigger-not-necessarily-better.
Ashton, Nick M. 2002. “Absence of Humans in Britain during the Last Interglacial Period (Oxygen Isotope Stage 5e).” Publications du CERP 8: 93–103.
Berger, Lee R., John Hawks, Darryl J. de Ruiter, Steven E. Churchill, Peter Schmid, Lucas K. Delezene, Tracy L. Kivell, et al. 2015. “Homo naledi, a New Species of the Genus Homo from the Dinaledi Chamber, South Africa.” eLife 4:e09560. https://doi.org/10.7554/eLife.09560.
Berger, Thomas D., and Erik Trinkaus. 1995. “Patterns of Trauma among the Neanderthals.” Journal of Archaeological Science 22 (6): 841–852.
Blangero, J., E.E. Quillen, M.A. Almeida, D.R. McKay, J.M. Peralta, S. Williams-Blangero, J.E. Curran, R. Duggirala, D.C. Glahn. 2014. “Genomic Reconstruction of Neandertal Brain Structure and Function.” Paper presented at the American Association of Physical Anthropologists Annual Meeting, Calgary, Alberta, Canada, 12 April 2014.
Bocherens, Hervé, Daniel Billiou, André Mariotti, Marylène Patou-Mathis, Marcel Otte, Dominique Bonjean, and Michel Toussaint. 1999. “Palaeoenvionmental and Palaeodietary Implications of Isotopic Biogeochemistry of Last Interglacial Neanderthal and Mammal Bones in Scladina Cave (Belgium).” Journal of Archaeological Science 26 (6): 599–607.
Bocquet-Appel, Jean-Pierre and Anna Degioanni. 2013. “Neanderthal Demographic Estimates.” Current Anthropology 54 (S8): S202–S213.
Boettger, Tatjana, Elena Yu. Novenko, Andrej A. Velichko, Olga K. Borisova, Konstantin V. Kremenetski, Stefan Knetsch, and Frank W. Junge. 2009. “Instability of Climate and Vegetation Dynamics in Central and Eastern Europe during the Final Stage of the Last Interglacial (Eemian, Mikulino) and Early Glaciation.” Quaternary International 207 (1-2): 137–144. https://doi.org/10.1016/j.quaint.2009.05.006.
Brophy, Juliet K., Marina C. Elliott, Darryl J. De Ruiter, Debra R. Bolter, Steven E. Churchill, Christopher S. Walker, John Hawks, and Lee R. Berger. 2021. “Immature Hominin Craniodental Remains from a New Locality in the Rising Star Cave System, South Africa.” PaleoAnthropology 1: 1–14. https://doi.org/10.48738/2021.iss1.64.
Browning, Sharon R., Brian L. Browning, Ying Zhou, Serena Tucci, and Josh M. Akey. 2018. “Analysis of Human Sequence Data Reveals Two Pulses of Archaic Denisovan Admixture.” Cell 173 (1): 53–61.
Chen, Fahu, Frido Welker, Chuan-Chou Shen, Shara E. Bailey, Inga Bergmann, Simon Davis, Huan Xia, et al. 2019. “A Late Middle Pleistocene Denisovan Mandible from the Tibetan Plateau.” Nature 569: 409–412. https://doi.org/10.1038/s41586-019-1139-x.
Churchill, Steven E. 1998. “Cold Adaptations, Heterochrony, and Neanderthals.” Evolutionary Anthropology 7 (2): 46–60.
Churchill, Steven E. 2006. “Bioenergetic Perspective on Neanderthal Thermoregulatory and Activity Budgets.” In Neanderthals Revisited: New Approaches and Perspectives, edited by K. Havarti and T. Harrison, pp. 113–134. Dordrecht, Germany: Springer.
Churchill, Steven E., Robert G. Franciscus, Hilary A. McKean-Peraza, Julie A. Daniel, and Brittany R. Warren. 2009. “Shanidar 3 Neanderthal Rib Puncture Wound and Paleolithic Weaponry.” Journal of Human Evolution 57 (2): 163–178.
Cook, Rebecca W., Antonio Vazzana, Rita Sorrentino, Stefano Benazzi, Amanda L. Smith, David S. Strait, and Justin A. Ledogar. 2021. “The Cranial Biomechanics and Feeding Performance of Homo floresiensis.” Interface Focus 11 (5). https://doi.org/10.1098/rsfs.2020.0083.
Dawson, James E., and Erik Trinkaus. 1997. “Vertebral Osteoarthritis of La Chapelle-Aux-Saints Neanderthal.” Journal of Archaeological Science 24 (11): 1015-1021. https://doi.org/10.1006/jasc.1996.0179.
Defleur, Alban R., and Emmanuel Desclaux. 2019. “Impact of the Last Interglacial Climate Change on Ecosystems and Neanderthals Behavior at Baume Moula-Guercy, Ardèche, France.” Journal of Archaeological Science 104: 114–124. https://doi.org/10.1016/j.jas.2019.01.002.
Degano, Ilaria, Sylvain Soriano, Paola Villa, Luca Pollarolo, Jeannette J. Lucejko, Zenobia Jacobs, Katerina Douka, Silvana Vitagliano, and Carlo Tozzi. 2019. “Hafting of Middle Paleolithic Tools in Latium (Central Italy): New Data from Fossellone and Sant’Agostino Caves.” PLoS ONE 14(10): 1–29. https://doi.org/10.1371/journal.pone.0213473.
Degioanni Anna, Chritophe Bonenfant, Sandrine Cabut, and Silvana Condemi. 2019. Living on the Edge: Was Demographic Weakness the Cause of Neanderthal Demise? PLoS ONE 14 (5): e0216742.
https://doi.org/10.1371/journal.pone.0216742.
Delpiano, Davide, Kristin Heasley, and Marci Peresani. 2018. “Assessing Neanderthal Land Use and Lithic Raw Material Management in Discoid Technology.” Journal of Anthropological Sciences 96: 89–110. https://doi.org/10.4436/jass.96006.
Demeter, Fabrice, Clément Zanolli, Kira E. Westaway, Renaud Joannes-Boyau, Phillippe Duringer, Mike W. Morley, Frido Welker, et al. 2022. “A Middle Pleistocene Denisovan Molar from the Annamite Chain of Northern Laos.” Nature Communications 13 (1): 2557. https://doi.org/10.7554/eLife.24231.
Dirks, Paul H. G. M., Eric M. Roberts, Hannah Hilbert-Wolf, Jan D. Kramers, John Hawks, Anthony Dosseto, Mathieu Duval, et al. 2017. “The Age of Homo naledi and Associated Sediments in the Rising Star Cave, South Africa.” eLife 6: e24231. https://doi.org/10.7554/eLife.24231.
el-Showk, Sedeer. 2019. “Neanderthal Clues to Brain Evolution in Humans.” Nature 571: S10–S11. https://doi.org/10.1038/d41586-019-02210-6.
Esteban, Irene, Curtis W. Marean, Erich C. Fisher, Panagiotis Karkanas, Dan Carbanes, and Rosa M. Albert. 2018. “Phytoliths as an Indicator of Early Modern Humans’ Plant-Gathering Strategies, Fire, Fuel, and Site-Occupation Intensity During the Middle Stone Age at Pinnacle Point 5-6 (South Coast, South Africa).” PLoS One 13 (6): 1–33.
Evteev, Andrej A., Alla A. Movsesian, and Alexandra N. Grosheva. 2017. “The Association between Mid-facial Morphology and Climate in Northeast Europe Differs from That in North Asia: Implications for Understanding the Morphology of Late Pleistocene Homo sapiens.” Journal of Human Evolution 107: 36–48. https://doi.org/10.1016/j.jhevol.2017.02.008.
Frayer, David W., Marina Lozano, Jose M. Bermudez de Castro, Eudald Carbonell, Juan-Luis Arsuaga, Jakov Radovcic, Ivana Fiore, and Luca Bondioli. 2012. “More Than 500,000 Years of Right-handedness in Europeans.” Laterality 17 (1): 51–69. https://doi.org/10.1080/1357650X.2010.529451.
Fu, Qiaomei, Mateja Hajdinjak, Oana Teodora Moldovan, Silviu Constantin, Swapan Mallick, Pontus Skoglund, Nick Patterson, et al. 2015. “An Early Modern Human from Romania with a Recent Neanderthal Ancestor.” Nature 524 (7564): 216. https://doi.org/10.1038/nature14558.
Gaudzinski-Windheuser, Sabine, Elizabeth S. Noack, Eduard Pop, Constantin Herbst, Johannes Pfleging, Jonas Buchli, Arne Jacob, et al. 2018. “Evidence for Close-Range Hunting by Last Interglacial Neanderthals.” Nature Ecology and Evolution 2: 1087–1092. https://doi.org/10.1038/s41559-018-0596-1.
Gilpin, William., Marcus W. Feldman, and Kenichi Aoki. 2016. “An Ecocultural Model Predicts Neanderthal Extinction through Competition with Modern Humans.” Proceedings of the National Academy of Sciences 113 (8): 2134–2139. https://doi.org/10.1073/pnas.1524861113.
Golovanova, Liubov. Vitaliena, Vladimir B. Doronichev, Naomi E. Cleghorn, Marianna A. Koulkova, Tatiana V. Sapelko, and M. Steven Shackley. 2010. “Significance of Ecological Factors in the Middle to Upper Paleolithic Transition.” Current Anthropology 51 (5): 655–691. https://doi.org.10.1086/656185.
Harrold, Francis B. 1980. “A Comparative Analysis of Eurasian Paleolithic Burials.” World Archaeology 12 (2): 195–211.
Hawks, John, Marina Elliott, Peter Schmid, Steven E. Churchill, Darryl J. de Ruiter, Eric M. Roberts, Hannah Hilbert-Wolf, et al. 2017. “New Fossil Remains of Homo naledi from the Lesedi Chamber, South Africa.” eLife 6: e24232. https://doi.org/10.7554/eLife.24232.
Henry, Amanda G., Alison S. Brooks, and Dolores R. Piperno. 2010. “Microfossils and Calculus Demonstrate Consumption of Plants and Cooked Foods in Neanderthal Diets (Shanidar III, Iraq; Spy I and II, Belgium).” Proceedings of the National Academy of Sciences USA 108 (2): 486–491. https://doi.org/10.1073/pnas.1016868108.
Houldcroft, Charlotte J., and Simon J. Underdown. 2016. “Neanderthal Genomics Suggests a Pleistocene Time Frame for the First Epidemiologic Transition.” American Journal of Physical Anthropology 160 (3): 379–388.
Huerta-Sánchez, Emilia, Xin Jin, Asan, Zhuoma Bianba, Benjamin M. Peter, Nicolas Vinckenbosch, Yu Liang, et al. 2014. “Altitude Adaptation in Tibetans Caused by Introgression of Denisovan-like DNA.” Nature 512 (7513): 194-197.
Jaouen, Klervia, Michael P. Richards, and Adeline Le Cabec. 2019. “Exceptionally High δ15N Values in Collagen Single Amino Acids Confirm Neanderthals as High-Trophic Level Carnivores.” Proceedings of the National Academy of Sciences 116 (11): 4928–4933. https://doi.org/10.1073/pnas.1814087116.
Kandel, Andrew W., Michael Bolus, Knut Bretzke, Angela A. Bruch, Miriam N. Haidle, Christine Hertler, and Michael Märker. 2015. “Increasing Behavioral Flexibility? An Integrative Macro-Scale Approach to Understanding the Middle Stone Age of Southern Africa.” Journal of Archaeological Method and Theory 23: 623–668. https://doi.org/10.1007/s10816-015-9254-y.
Kortlandt, Adriaan. 2002. “Neanderthal Anatomy and the Use of Spears.” Evolutionary Anthropology 11 (5): 183–184.
Kruger, A., P. Randolph-Quinney, and M. Elliott. 2016. “Multimodal Spatial Mapping and Visualization of Dinaledi Chamber and Rising Star Cave.” South African Journal of Science 112 (5–6). https://doi.org/10.17159/sajs.2016/20160032.
Lieberman, Philip, and Edmund S. Crelin. 1971. “On the Speech of Neanderthal Man.” Linguistic Inquiry 2 (2): 203–222.
Mendez, Fernando L., G. David Poznik, Sergi Castellano, and Carlos D. Bustamante. 2016. “The Divergence of Neanderthal and Modern Human Y Chromosomes.” American Journal of Human Genetics 98 (4): 728–734.
Mora-Bermúdez, Felipe, Philipp Kanis, Dominik Macak, Jula Peters, Ronald Naumann, Lei Xing, Mihail Sarov, et al. 2022. “Longer Metaphase and Fewer Chromosome Segregation Errors in Modern Human Than Neanderthal Brain Development.” Science Advances 8 (30). https://doi.org/10.1126/sciadv.abn7702.
Nicholson, Christopher M. 2017. “Eemian Paleoclimate Zones and Neanderthal Landscape Use: A GIS Model of Settlement Patterning during the Last Interglacial.” Quaternary International 438 (B): 144–157. https://doi.org/10.1016/j.quaint.2017.04.023.
Nobel Prize, The. 2022. “Press Release: The Nobel Prize in Physiology or Medicine 2022.” Nobelförsamlingen The Nobel Assembly at Karolinska Institutet. Press release, November 3, 2022. https://www.nobelprize.org/prizes/medicine/2022/press-release/.
Nowell, April. 2016. “Childhood, Play and the Evolution of Cultural Capacity in Neanderthals and Modern Humans.” In The Nature of Culture: Based on an Interdisciplinary Symposium “The Nature of Culture,” Tübingen, Germany, edited by M. N. Haidle, N. J. Conard, and M. Bolus, 87–97. Heidelberg, Germany: Springer. https://doi.org.10.1007/978-94-017-7426-0_9.
Parkington, John. 2003. “Middens and Moderns: Shellfishing and the Middle Stone Age of the Western Cape, South Africa.” South African Journal of Science 99 (5–6): 243–274.
Pearce, Eiluned, Chris Stringer, and R. I. M. Dunbar. 2013. “New Insights into Differences in Brain Organizations between Neanderthals and Anatomically Modern Humans.” Proceedings of the Royal Society B: Biological Sciences 280 (1758): 20130168. https://doi.org/10.1098/rspb.2013.0168.
Pomeroy, Emma, Paul Bennett, Chris O. Hunt, Tim Reynolds, Lucy Farr, Marine Frouin, James Holman, Ross Lane, Charles French, and Graeme Barker. 2020. “New Neanderthal Remains Associated with the ‘Flower Burial’ at Shanidar Cave.” Antiquity 94 (373): 11–26. https://doi.org/10.15184/aqy.2019.207.
Rae, Todd, Thomas Koppe, and Chris B. Stringer. 2011. “The Neanderthal Face Is Not Cold-Adapted.” Journal of Human Evolution 60 (2): 234–239. https://doi.org/10.1016/j.jhevol.2010.10.003.
Reich, David, Richard E. Green, Martin Kircher, Johannes Krause, Nick Patterson, Eric Y. Durand, Bence Viola, et al. 2010. “Genetic History of an Archaic Hominin Group from Denisova Cave in Siberia.” Nature 468: 1053–1060. https://doi.org/10.1038/nature09710.
Richards, Michael P., Paul B. Pettit, Erik Trinkaus, Fred H. Smith, Maja Paunović, and Ivor Karavanić. 2000. “Neanderthal Diet at Vindija and Neanderthal Predation: The Evidence from Stable Isotopes.” Proceedings of the National Academy of Sciences 97 (13): 7663–7666.
https://doi.org/10.1073/pnas.120178997.
Richter, Jürgen, 2006. “Neanderthals in Their Landscape: Neanderthals in Europe.” In Proceedings of the International Conference held in the Gallo-Roman Museum in Tongeren, ERAUL 117, edited by B. Demarsin and M. Otte, 17–32. Köln: Universität zu Köln.
Roksandic, Mirjana, Predrag Radovic, Xiu-Jie Wu, and Christopher J. Bae. 2021. “Resolving the ‘Muddle in the Middle’: The Case for Homo bodoensis.” Evolutionary Anthropology: Issues, News, and Reviews, 31 (1). https://doi.org/10.1002/evan.21929.
Slon, Viviane, Fabrizio Mafessoni, Benjamin Vernot, Cesare de Filippo, Steffi Grote, Bence Viola, Mateja Hajdinjak, et al. 2018. “The Genome of the Offspring of a Neanderthal Mother and a Denisovan Father.” Nature 561 (7721): 113–116.
Smith, Tanya M., Paul Tafforeau, Donald J. Reid, Joanne Pouech, Vincent Lazzari, John P. Zermeno, Debbi Guatelli-Steinberg, et al. 2010. “Dental Evidence for Ontogenetic Differences Between Modern Humans and Neanderthals.” Proceedings for the National Academy of Sciences 107 (49): 20923–20928.
Staubwasser, Michael, Virgil Drăguʂin, Bogdan P. Onac, Sergey Assonov, Vasile Ersek, Dirk L. Hoffman, and Daniel Veres. 2018. “Impact of Climate Change on the Transition of Neanderthals to Modern Humans in Europe.” Proceedings of the National Academy of Sciences 115 (37): 9116–9121. https://doi.org/10.1073/pnas.1808647115.
Stewart, T. D. 1977. “The Neanderthal Skeletal Remains from Shanidar Cave, Iraq: A Summary of Findings to Date.” Proceedings of the American Philosophical Society 121 (2): 121–165.
Stolarczyk, Regine E., and Patrick Schmidt. 2018. “Is Early Silcrete Heat Treatment a New Behavioural Proxy in the Middle Stone Age?” PLoS One 13 (10): 1–21.
Trinkaus, E. 1985. “Pathology and Posture of the La-Chapelle-aux-Saints Neanderthal.” American Journal of Physical Anthropology 67 (1): 19–41.
Trujillo Cleber A., Edward S. Rice, Nathan K. Schaefer, Isaac A. Chaim, Emily C. Wheeler, Assael A. Madrigal, Justin Buchanan, et al. 2021. “Reintroduction of the Archaic Variant of NOVA1 in Cortical Organoids Alters Neurodevelopment.” Science 371 (6530): eaax2537. https://doi.org/10.1126/science.aax2537.
Tucci, Serena, Samuel H. Vohr, Rajiv C. McCoy, Benjamin Vernot, Matthew R. Robinson, Chiara Barbieri, Brad J. Nelson, et al. 2018. “Evolutionary History and Adaptation of a Human Pygmy Population of Flores Island, Indonesia.” Science 361 (6401): 511–516.
Van Andel, T. H., and P. C. Tzedakis. 1996. “Paleolithic Landscapes of Europe and Environs, 150,000–25,000 Years Ago: An Overview.” Quaternary Science Reviews 15 (5–6): 481–500.
Venner, Stephen J. 2018. “A New Estimate for Neanderthal Energy Expenditure.” CUNY Academic Works.
Vernot, Benjamin, Serena Tucci, Janet Kelso, Joshua G. Schraiber, Aaron B. Wolf, Rachel M. Gittelman, Michael Danneman, et al. 2016. “Excavating Neanderthal and Denisovan DNA from the Genomes of Melanesian Individuals.” Science 352 (6282): 235–239.
Weaver, T. D., and J. Hublin. 2009. “Neanderthal Birth Canal Shape and the Evolution of Human Childbirth.” Proceedings of the National Academy of Sciences 106 (20): 8151–8156.
Wiẞing, Christoph, Hélène Rougier, Isabelle Crevecoer, Mietje Germonpré, Yuichi Naito, Patrick Semal, and Hervé Bocherens. 2015. “Isotopic Evidence for Dietary Ecology of Late Neanderthals in Northwestern Europe.” Quaternary International 411 (A): 327–345. https://doi.org/10.1016/j.quaint.2015.09.091.
Wong, Kate. 2015. “Neanderthal Minds.” Scientific American (January): 312(2): 36-43. https://doi.org/10.1038/scientificamerican0215-36.
Zhang, X. L., B. B. Ha, S. J. Wang, Z. J. Chen, J. Y. Ge, H. Long, W. He, et al. 2018. “The Earliest Human Occupation of the High-Altitude Tibetan Plateau 40 Thousand to 30 Thousand Years Ago.” Science 362 (6418): 1049–1051. https://doi.org/10.1126/sciadv.add5582.
Zilhão João, Diego E. Angelucci, Ernestina Badal-García, Francesco d’Errico, Floréal Daniel, Laure Dayet, Katerina Douka, et al. 2010. “Symbolic Use of Marine Shells and Mineral Pigments by Iberian Neandertals.” Proceedings of the National Academy of Sciences 107 (3): 1023–1028. https://doi.org/10.1073/pnas.0914088107.
Zollikofer, Christopher Peter Edwards, and Marcia Silvia Ponce de León. 2013. “Pandora’s Growing Box: Inferring the Evolution and Development of Hominin Brains from Endocasts.” Evolutionary Anthropology 22 (1): 20–33. https://doi.org/10.1002/evan.21333.
Acknowledgments
The authors would like to extend their thanks to Cassandra Gilmore and Anna Goldfield for thoughtful and insightful suggestions on the first edition of this chapter.
Applying negative judgments to other cultures based on comparison to one’s own.
A way of thinking that assumes humans are the most important species and leads to interpreting the world always through a human lens. Species-centric science and thought.
A glacial period, or time when a large portion of the world is covered by glaciers and ice sheets.
A warmer period between two glacial time periods.
Single-celled marine organisms with shells.
Round-shaped, like a globe.
A face that is flatter.
The opening for the nose visible on a skull. Often pear- or heart-shaped.
A forward projection of the nose or the middle facial region. Usually associated with Neanderthals.
A space behind the last molar and the end of the jaw. This is a feature present only on Neanderthals. It also occurs through cultural modification in modern humans who have had their third molars, or wisdom teeth, removed.
A prominent bulge or projection on the back of the skull, specifically the occipital bone. This is a feature present only on Neanderthal skulls.
Small holes on the maxilla bone of the face that allows nerves and blood to reach the skin.
The stone tool industry of Neanderthals and their contemporaries in Africa and Western Asia. Mousterian tools are known for a diverse set of flake tools, which is different from the large bifacial tools of the Acheulean industry.
A distinctive technique of stone tool manufacturing used by Archaic Homo sapiens, including Neanderthals. The technique involves the preparation of a core and striking edges off in a regular fashion around the core. Then a series of similarly sized pieces can be removed, which can then be turned into different tools.
The outside, or rough outer covering, of a rock. Usually the cortex is removed during the process of stone tool creation.
A handle. Also used as a verb—to attach a handle to an item, such as a stone tool.
Fetal position, in which the legs are drawn up to the middle of the body and the arms are drawn toward the body center. Intentional burials are often found in the flexed body position.
Items included with a body at burial. Items may signify occupation or hobbies, social status, or level of importance in the community, or they may be items believed necessary for the afterlife.
A form of dwarfism that occurs when a limited geographic region, such as an island, causes a large-bodied animal to be selected for a smaller body size.
