17 Social and Biopolitical Dimensions of Evolutionary Thinking
Jonathan Marks, Ph.D., University of North Carolina at Charlotte
Adam P. Johnson, M.A., University of North Carolina at Charlotte/University of Texas at San Antonio
This chapter is an adaptation of “Chapter 2: Evolution” by Jonathan Marks. In Explorations: An Open Invitation to Biological Anthropology, first edition, edited by Beth Shook, Katie Nelson, Kelsie Aguilera, and Lara Braff, which is licensed under CC BY-NC 4.0.
Learning Objectives
- Explain the relationship among genes, bodies, and organismal change.
- Discuss the shortcomings of simplistic understandings of genetics.
- Describe what is meant by the “biopolitics of heredity.”
- Discuss issues caused by misuse of ideas about adaptations and natural selection.
- Examine and correct myths about evolution.
The Human Genome Project, an international initiative launched in 1990, sought to identify the entire genetic makeup of our species. For many scientists, it meant trying to understand the genetic underpinnings of what made humans uniquely human. James Watson, a codiscoverer of the helical shape of DNA, wrote that “when finally interpreted, the genetic messages encoded within our DNA molecules will provide the ultimate answers to the chemical underpinnings of human existence” (Watson 1990, 248). The underlying message is that what makes humans unique can be found in our genes. The Human Genome Project hoped to find the core of who we are and where we come from.
Despite its lofty goal, the Human Genome Project—even after publishing the entire human genome in January 2022—could not fully account for the many factors that contribute to what it is to be human. Richard Lewontin, Steven Rose, and Leon Kamin (2017) argue that genetic determinism of the sort assumed by the Human Genome Project neglects other essential dimensions that contribute to the development and evolution of human bodies, not to mention the role that culture plays. They use an apt metaphor of a cake to illustrate the incompleteness of reductive models. Consider the flavor of a cake and think of the ingredients listed in the recipe. The recipe includes ingredients such as flour, sugar, shortening, vanilla extract, eggs, and milk. Does raw flour taste like cake? Does sugar, vanilla extract, or any of the other ingredients taste like cake? They do not, and knowing the individual flavors of each ingredient does not tell us much about what cake tastes like. Even mixing all of the ingredients in the correct proportions does not get us cake. Instead, external factors such as baking at the right temperature, for the right amount of time, and even the particularities of our evolved sense of taste and smell are all necessary components of experiencing the cake.
Lewontin, Rose, and Kamin (2017) argue that the same is true for humans and other organisms.
Knowing everything about cake ingredients does not allow us to fully know cake. Equally so, knowing everything about the genes found in our DNA does not allow us to fully know humans. Different, interacting levels are implicated in the development and evolution of all organisms, including humans. Genes, the structure of chromosomes, developmental processes, epigenetic tags, environmental factors, and still-other components all play key roles such that genetically reductive models of human development and evolution are woefully inadequate.
The complex interactions across many levels—genetic, developmental, and environmental—explain why we still do not know how our one-dimensional DNA nucleotide sequence results in a four-dimensional organism. This was the unfulfilled promise of the inception of the Human Genome Project in the 1980s and 1990s: the project produced the complete DNA sequence of a human cell in the hopes that it would reveal how human bodies are built and how to cure them when they are built poorly. Yet, that information has remained elusive. Presumably, the knowledge of how organisms are produced from DNA sequences will one day permit us to reconcile the discrepancies between patterns in anatomical evolution and molecular evolution.
In this chapter, we will consider multilevel evolution and explore evolution as a complex interaction between genetic and epigenetic factors as well as the environments in which organisms live. Next, we will examine the biopolitical nature of human evolution. We will then investigate problems that arise from attributing all traits to an adaptive function. Finally, we will address common misconceptions about evolution. The goal of this chapter is to provide you with the necessary toolkit for understanding the molecular, anatomical, and political dimensions of evolution.
Evolution Happens at Multiple Levels
Following Richard Dawkins’s publication of The Selfish Gene in 1976, the scientific imagination was captured by the potential of genomics to reveal how genes are copied by Darwinian selection. Dawkins argues that the genes in individuals that contribute to greater reproductive success are the units of selection. His conception of evolution at the molecular level undercuts the complex interactions between organisms and their environments, which are not expressed genomically but are nevertheless key drivers in evolution.
By the 1980s, the acknowledgment among most biologists that even though genes construct bodies, genes and bodies evolve at different rates and with distinct patterns. This realization led to a renewed focus on how bodies change. The Evolutionary Synthesis of the 1930s–1970s had reduced organisms to their genotypes and species to their gene pools, which provided valuable insights about the processes of biological change, but it was only a first approximation. Animals are in fact reactive and adaptable beings, not passive and inert genotypes. Species are clusters of socially interacting and reproductively compatible organisms.

Once we accept that evolutionary change is fundamentally genetic change, we can ask: How do bodies function and evolve? How do groups of animals come to see one another as potential mates or competitors for mates, as opposed to just other creatures in the environment? Are there evolutionary processes that are not explicable by population genetics? These questions—which lead us beyond reductive assumptions—were raised in the 1980s by Stephen Jay Gould, the leading evolutionary biologist of the late 20th century (see: Gould 2003; 1996).
Gould spearheaded a movement to identify and examine higher-order processes and features of evolution that were not adequately explained by population genetics. For example, extinction, which was such a problem for biologists of the 1600s, could now be seen as playing a more complex role in the history of life than population genetics had been able to model. Gould recognized that there are two kinds of extinctions, each with different consequences: background extinctions and mass extinctions. Background extinctions are those that reflect the balance of nature, because in a competitive Darwinian world, some things go extinct and other things take their place. Ecologically, your species may be adapted to its niche, but if another species comes along that’s better adapted to the same niche, eventually your species will go extinct. It sucks, but it is the way of all life: you come into existence, you endure, and you pass out of existence. But mass extinctions are quite different. They reflect not so much the balance of nature as the wholesale disruption of nature: many species from many different lineages dying off at roughly the same time—presumably as the result of some kind of rare ecological disaster. The situation may not be survival of the fittest as much as survival of the luckiest. The result, then, would be an ecological scramble among the survivors. Having made it through the worst, the survivors could now simply divide up the new ecosystem amongst themselves, since their competitors were gone. Something like this may well have happened about 65 million years ago, when a huge asteroid hit the Yucatan Peninsula, which mammals survived but dinosaurs did not (Figure 17.1). Something like this may be happening now, due to human expansion and environmental degradation. Note, though, that there is only a limited descriptive role here for population genetics: the phenomena we are describing are about organisms and species in ecosystems.
Another question involved the disconnect between properties of species and the properties of gene pools. For example, there are upwards of 15 species of gibbons but only two species of chimpanzees. Why? There are upwards of 20 species of guenons but fewer than ten of baboons. Why? Are there genes for that? It seems unlikely. Gould suggested that species, as units of nature, might have properties that are not reducible to the genes in their cells. For example, rates of speciation and extinction might be properties of their ecologies and histories rather than their genes. Thus, relationships between environmental contexts and variability within a species result in degrees of resistance to extinction and affect the frequency and rates at which clades diversify (Lloyd and Gould 1993). Consistent biases of speciation rates might well produce patterns of macroevolutionary diversity that are difficult to explain genetically and better understood ecologically. Gould called such biases in speciation rates species selection—a higher-order process that invokes competition between species, in addition to the classic Darwinian competition between individuals.
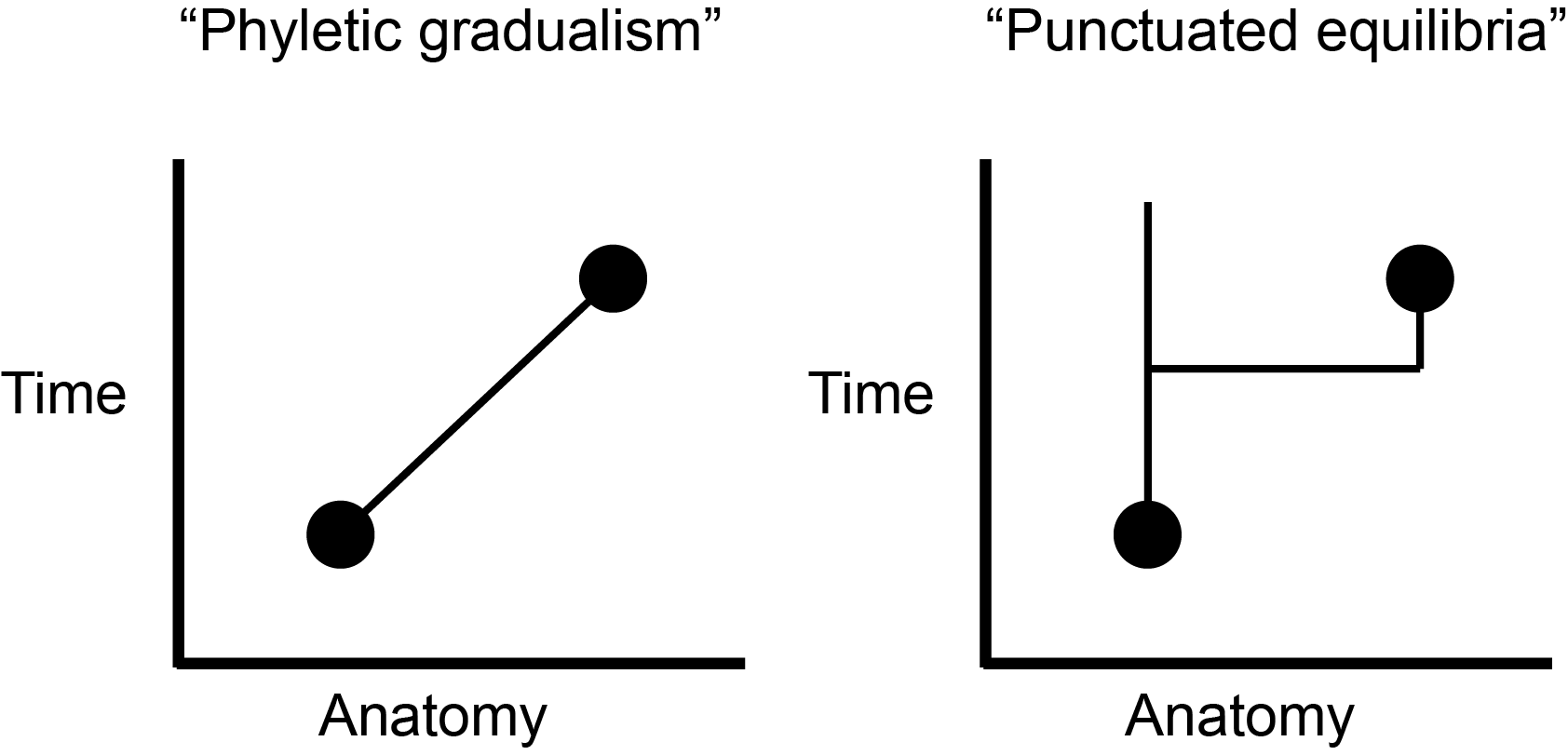
One of Gould’s most important studies involved the very nature of species. In the classical view, a species is continually adapting to its environment until it changes so much that it is a different species than it was at the beginning of this sentence (Eldredge and Gould 1972). That implies that the species is a fundamentally unstable entity through time, continuously changing to fit in. But suppose, argued Gould along with paleontologist Niles Eldredge, a species is more stable through time and only really adapts during periods of ecological instability and change. Then we might expect to find in the fossil record long equilibrium periods—a few million years or so—in which species don’t seem to change much, punctuated by relatively brief periods in which they change a bit and then stabilize again as new species. They called this idea punctuated equilibria. The idea helps to explain certain features of the fossil record, notably the existence of small anatomical “gaps” between closely related fossil forms (Figure 17.2). Its significance lies in the fact that although it incorporates genetics, punctuated equilibria is not really a theory of genetics but one of types bodies in deep time.
Punctuated equilibria is seen across taxa, with long periods in the fossil record representing little phenotypic change. These periods of stability are disrupted by shorter periods of rapid adaptation, the process through which populations of organisms become suited to living in their environments. Phenotypic changes are often coupled with drastic climatic or ecological changes that affect the milieu in which organisms live. For example, throughout much of hominin evolutionary history, brain size was closely associated with body size and thus remained mostly stable. However, changes occurred in average hominin brain size at around 100 thousand years ago, 1 million years ago, and 1.8 million years ago. Several hypotheses have been put forth to explain these changes, including unpredictability in climate and environment (Potts 1998), social development (Barton 1996), and the evolution of language (Deacon 1998). Evidence from the fossil record, paleoclimate models, and comparative anatomy suggests that the changes observed in hominin lineage result from biocultural processes—that is, the coalescence of environmental and cultural factors that selected for larger brains (Marks 2015; Shultz, Nelson, and Dunbar 2012).
In response to the call for a theory of the evolution of form, the field of evo-devo—the intersection of evolutionary and developmental biology—arose. The central focus here is on how changes in form and shape arise. An embryo matures by the stimulation of certain cells to divide, forming growth fields. The interactions and relationships among these growth fields generate the structures of the body. The hox genes that regulate these growth fields turn out to be highly conserved across the animal kingdom. This is because they repeatedly turn on and off the most basic genes guiding the animal’s development, and thus any changes to them would be catastrophic. Indeed, these genes were first identified by manipulating them in fruit flies, such that one could produce a bizarre mutant fruit fly that grew a pair of legs where its antennae were supposed to be (Kaufman, Seeger, and Olsen 1990).
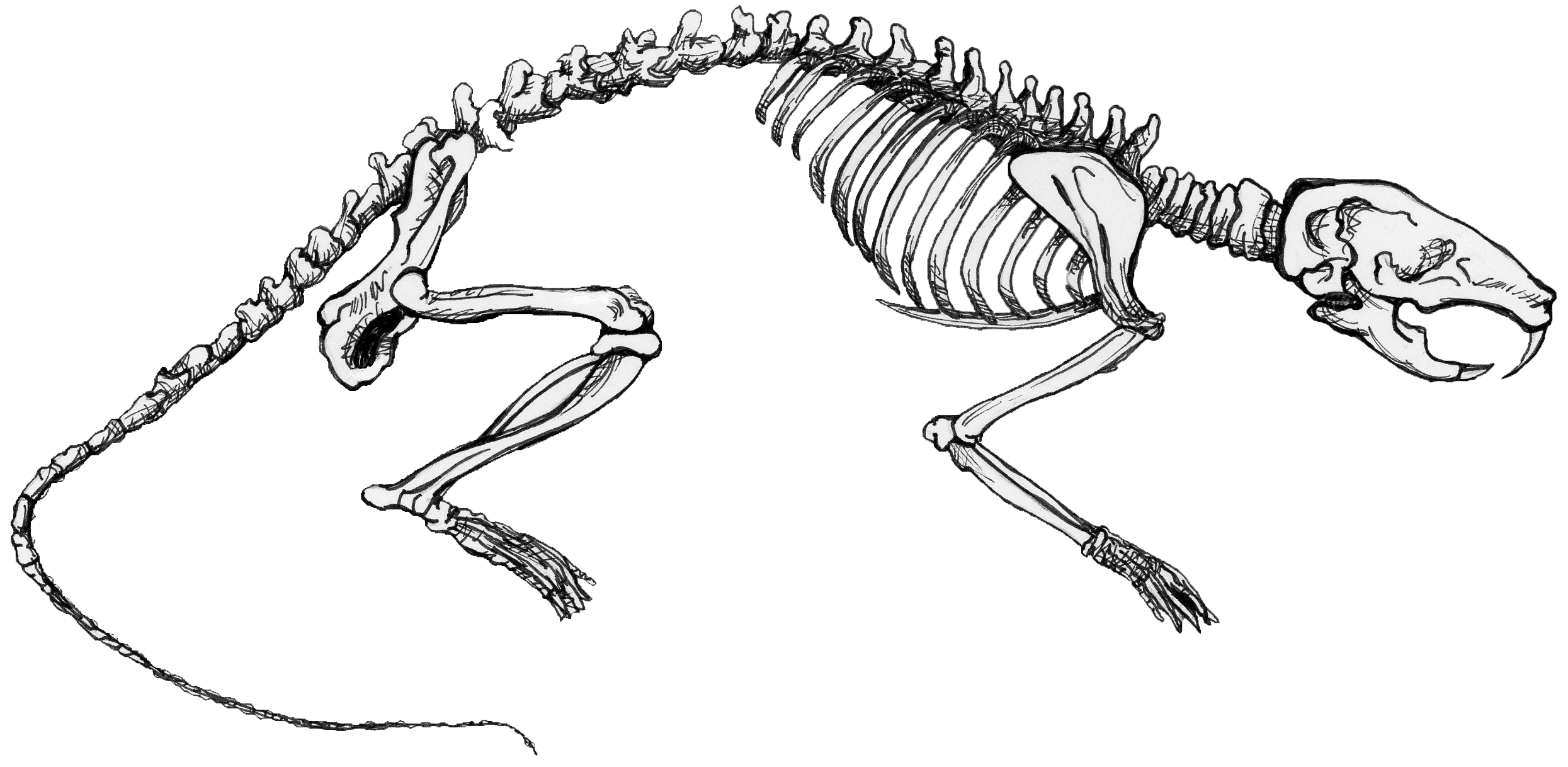
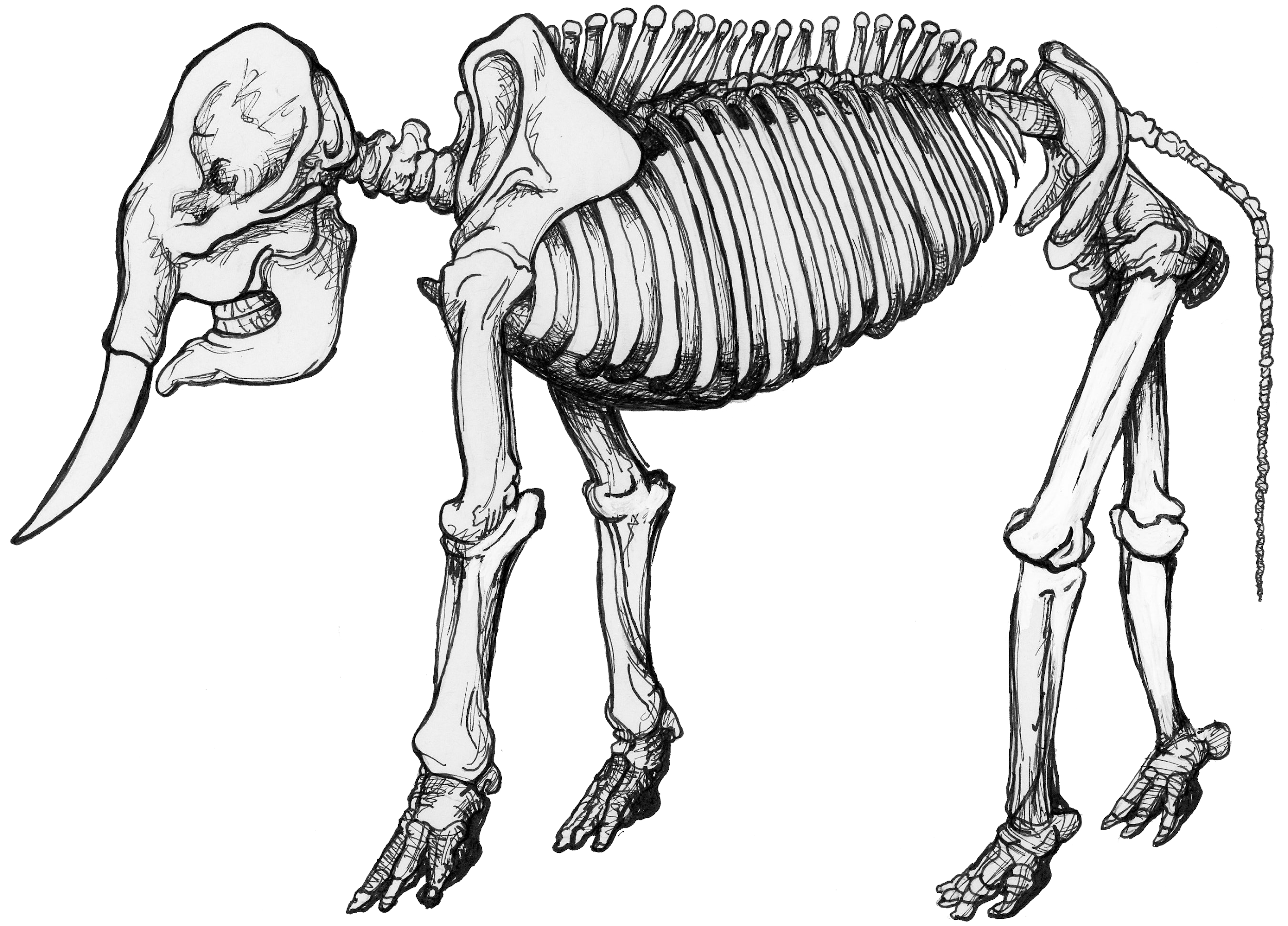
Certain genetic changes can alter the fates of cells and the body parts, while other genetic changes can simply affect the rates at which neighboring groups of cells grow and divide, thus producing physical bumps or dents in the developing body. The result of altering the relationships among these fields of cellular proliferation in the growing embryo is allometry, or the differential growth of body parts. As an animal gets larger—either over the course of its life or over the course of macroevolution—it often has to change shape in order to live at a different size. Many important physiological functions depend on properties of geometric area: the strength of a bone, for example, is proportional to its cross-sectional area. But area is a two-dimensional quality, while growing takes place in three dimensions—as an increase in mass or volume. As an animal expands, its bones necessarily weaken, because volume expands faster than area does. Consequently a bigger animal has more stress on its bones than a smaller animal does and must evolve bones even thicker than they would be by simply scaling the animal up proportionally. In other words, if you expand a mouse to the size of an elephant, it will nevertheless still have much thinner bones than the elephant does. But those giant mouse bones will unfortunately not be adequate to the task. Thus, a giant mouse would have to change aspects of its form to maintain function at a larger size (see Figure 17.3).
Physiologically, we would like to know how the body “knows” when to turn on and off the genes that regulate growth to produce a normal animal. Evolutionarily, we would like to know how the body “learns” to alter the genetic on/off switch (or the genetic “slow down/speed up” switch) to produce an animal that looks different. Moreover, since organisms differ from one another, we would like to know how the developing body distinguishes a range of normal variation from abnormal variation. And, finally, how does abnormal variation eventually become normal in a descendant species?
Taking up these questions, Gould invoked the work of a British geneticist named Conrad H. Waddington, who thought about genetics in less reductive ways than his colleagues. Rather than isolate specific DNA sites to analyze their function, Waddington instead studied the inheritance of an organism’s reactivity—its ability to adapt to the circumstances of its life. In a famous experiment, he grew fruit fly eggs in an atmosphere containing ether. Most died, but a few survived somehow by developing a weird physical feature: a second thorax with a second pair of wings. Waddington bred these flies and soon developed a stable line of flies who would reliably develop a second thorax when grown in ether. Then he began to lower the concentration of ether, while continuing to selectively breed the flies that developed the strange appearance. Eventually he had a line of flies that would stably develop the “bithorax” phenotype–the suite of traits of an organism–even when there was no ether; it had become the “new normal.” The flies had genetically assimilated the bithorax condition.
Waddington was thus able to mimic the inheritance of acquired characteristics: what had been a trait stimulated by ether a few generations ago was now a normal part of the development of the descendants. Waddington recognized that while he had performed a selection experiment on genetic variants, he had not selected for particular traits. Rather, he helped produce the physiological tendency to develop particular traits when appropriately stimulated. He called that tendency plasticity and its converse, the tendency to stay the same even under weird environmental circumstances, canalization. Waddington had initially selected for plasticity, the tendency to develop the bithorax phenotype under weird conditions, and then, later, for canalization, the developmental normalization of that weird physical trait. Although Waddington had high stature in the community of geneticists, evolutionary biologists of the 1950s and 1960s regarded him with suspicion because he was not working within the standard mindset of reductionism, which saw evolution as the spread of genetic variants that coded for favorable traits. Both Waddington and Gould resisted contemporary intellectual paradigms that favored reductive accounts of evolutionary processes. They conceived of evolution as an emergent process in which many external factors (e.g. climate, environment, predation) and internal factors (e.g., genotypes, plasticity, canalization) coalesce to produce the evolutionary trends that we observe in the fossil record and our genome.
While Gould and Waddington both looked beyond the genome to understand evolution, the Human Genome Project—an international project with the goal of identifying each base pair in the human genome in the 1990s—generated a great deal of public interest in analyzing the human DNA sequence from the standpoint of medical genetics. Some of the rhetoric aimed to sell the public on investing a lot of money and resources in sequencing the human genome in order to show the genetic basis of heritable traits, cure genetic diseases, and learn what it means ultimately to be biologically human. However, the Human Genome Project was not actually able to answer those questions through the use of genetics alone, and thus a broader, more holistic account was required.
This holistic account came from decades of research in human biology and anthropology, which understood the human body as highly adaptable, dynamic, and emergent. For example, in the early 20th century, anthropologist Franz Boas measured the skulls of immigrants to the U.S., revealing that environmental, not merely genetic, factors affected skull shape. The growing human body adjusts itself to the conditions of life, such as diet, sunshine, high altitude, hard labor, population density, how babies are carried—any and all of which can have subtle but consistent effects upon its development. There can thus be no normal human form, only a context-specific range of human forms.
However, what the human biologists called human adaptability, evolutionary biologists called developmental plasticity, and evidence quickly began to mount for its cause being epigenetic modifications to DNA. Epigenetic modifications are changes to how genes are used by the body (as opposed to changes in the DNA sequences; see Chapter 3). Scientific interest shifted from the focus of the Human Genome Project to the ways that bodies are made by evolutionary-developmental processes, including epigenetics. What is meant by “epigenetic modification”? Evolution is about how descendants diverge from their ancestors. Inheritance from parent to offspring is still critical to this process, which occurs through genetic recombination: the pairing of homologous chromosomes and sharing of genetic material during meiosis (see Chapter 3). However, in the 21st century, the link between evolution and inheritance has broadened with a clearer understanding of how environmental and developmental factors shape bodies and the expression of genes, including epigenetic inheritance patterns. While offspring inherit their genes through random assortment during meiosis, environmental factors also shape how genes are used. When these epigenetic modifications occur in germ cells, they can be passed onto offspring. In these cases, there is no change in the DNA sequence but rather in how genes are used by the body due to DNA methylation and the structure of chromosomes due to histone acetylation (see Chapter 3).
In addition, we now recognize that evolution is affected by two other forms of intergenerational transmission and inheritance (in addition to genetics and epigenetics). These forms include behavioral variation and culture. That is, behavioral information can be transmitted horizontally (intragenerationally), permitting more rapid ways for organisms to adjust to the environment. And, then there is the fourth mode of transmission: the cultural or symbolic mode. Humans are the only species that horizontally transmits an arbitrary set of rules to govern communication, social interaction, and thought. This shared information is symbolic and has resulted in what we recognize as “culture”: locally emergent worlds of names, words, pictures, classifications, revered pasts, possible futures, spirits, dead ancestors, unborn descendants, in-laws, politeness, taboo, justice, beauty, and story, all accompanied by practices and a material world of tools.
Consequently our contemporary ideas about evolution see the evolutionary processes as hierarchically organized and not restricted to the differential transmission of DNA sequences into the next generation. While that is indeed a significant part of evolution, the organism and species are nevertheless crucial to understanding how those DNA sequences get transmitted. Further, the transmission of epigenetic, behavioral, and symbolic information play a complex role in perpetuating our genes, bodies, and species. In the case of human evolution, one can readily see that symbolic information and cultural adaptation are far more central to our lives and our survival today than DNA and genetic adaptation. It is thus misleading to think of humans passively occupying an environmental niche. Rather, humans are actively engaged in constructing our own niches, as well as adapting to them and using them to adapt. The complex interplay between a species and its active engagement in creating its own ecology is known as niche construction. If we understand natural selection–the process by which populations adapt to their specific environments–as the effects that environmental context has on the reproductive success of organisms, then niche construction is the process through which organisms shape their own selective pressures.
The Biopolitics of Heredity
“Science isn’t political” is a sentiment that you have likely heard before. Science is supposed to be about facts and objectivity. It exists, or at least ought to, outside of petty human concerns. However, the sorts of questions we ask as scientists, the problems we choose to study, the categories and concepts we use, who gets to do science, and whose work gets cited are all shaped by culture. Doing science is a political act. This fact is markedly true for human evolution. While it is easier to create intellectual distance between us and fruit flies and viruses, there is no distance when we are studying ourselves. The hardest lesson to learn about human evolution is that it is intensely political. Indeed, to see it from the opposite side, as it were, the history of creationism—the belief that the universe was divinely created around 6,000 years ago—is essentially a history of legal decisions. For instance, in Tennessee v. John T. Scopes (1925), a schoolteacher was prosecuted for violating a law in Tennessee that prohibited the teaching of human evolution in public schools, where teachers were required by law to teach creationism.
More recently, legal decisions aimed at legislating science education have shaped how students are exposed to evolutionary theory. For instance, McLean v. Arkansas (1982) dispatched “scientific creationism” by arguing that the imposition of balanced teaching of evolution and creationism in science classes violates the Establishment Clause, separating church and state. Additionally, Kitzmiller v. Dover (Pennsylvania) Area School District (2005) dispatched the teaching of “intelligent design” in public school classrooms as it was deemed to not be science. In some cases, people see unbiblical things in evolution, although most Christian theologians are easily able to reconcile science to the Bible. In other cases, people see immoral things in evolution, although there is morality and immorality everywhere. And some people see evolution as an aspect of alt-religion, usurping the authority of science in schools to teach the rejection of the Christian faith, which would be unconstitutional due to the protected separation of church and state.
Clearly, the position that politics has nothing to do with science is untenable. But is the politics in evolution an aberration or is it somehow embedded in science? In the early 20th century, scientists commonly promoted the view that science and politics were separate: science was seen as a pure activity, only rarely corrupted by politics. And yet as early as World War I, the politics of nationalism made a hero of the German chemist Fritz Haber for inventing poison gas. And during World War II, both German doctors and American physicists, recruited to the war effort, helped to end many civilian lives. Therefore, we can think of the apolitical scientist as a self-serving myth that functions to absolve scientists of responsibility for their politics. The history of science shows how every generation of scientists has used evolutionary theory to rationalize political and moral positions. In the very first generation of evolutionary science, Darwin’s Origin of Species (1859) is today far more readable than his Descent of Man (1871). The reason is that Darwin consciously purged The Origin of Species of any discussion of people. And when he finally got around to talking about people, in The Descent of Man, he simply imbued them with the quaint Victorian prejudices of his age, and the result makes you cringe every few pages. There is plenty of politics in there—sexism, racism, and colonialism—because you cannot talk about people apolitically.
One immediate faddish deduction from Darwinism, popularized by Herbert Spencer (1864) as “survival of the fittest,” held that unfettered competition led to advancement in nature and to human history. Since the poor were purported losers in that struggle, anything that made their lives easier would go against the natural order. This position later came to be known ironically as “Social Darwinism.” Spencer was challenged by fellow Darwinian Thomas Huxley (1863), who agreed that struggle was the law of the jungle but observed that we don’t live in jungles anymore. The obligation to make lives better for others is a moral, not a natural, fact. We simultaneously inhabit a natural universe of descent from apes and a moral universe of injustice and inequality, and science is not well served by ignoring the latter.
Concurrently, the German biologist Ernst Haeckel’s 1868 popularization of Darwinism was translated into English a few years later as The History of Creation. As we saw earlier, Haeckel was determined to convince his readers that they were descended from apes, even in the absence of fossil evidence attesting to it. When he made non-Europeans into the missing links that connected his readers to the apes, and depicted them as ugly caricatures, he knew precisely what he was doing. Indeed, even when the degrading racial drawings were deleted from the English translation of his book, the text nevertheless made his arguments quite clear. And a generation later, when the Americans had not yet entered the Great War in 1916, a biologist named Vernon Kellogg visited the German High Command as a neutral observer and found that the officers knew a lot about evolutionary biology, which they had gotten from Haeckel and which rationalized their military aggressions. Kellogg went home and wrote a bestseller about it, called Headquarters Nights (1917). World War I would have been fought with or without evolutionary theory, but as a source of scientific authority, evolution—even if a perversion of the Darwinian theory—had very quickly attained global geopolitical relevance.
Oftentimes, politics in evolutionary science is subtle, due to the pervasive belief in the advancement of science. We recognize the biases of our academic ancestors and modify our scientific stories accordingly. But we can never be free of our own cultural biases, which are invisible to us, as much as our predecessors’ biases were invisible to them. In some cases, the most important cultural issues resurface in different guises each generation, like scientific racism. Scientific racism is the recruitment of science for the evil political ends of racism, and it has proved remarkably impervious to evolution. Before Darwin, there was creationist scientific racism, and after Darwin, there was evolutionist scientific racism. And there is still scientific racism today, self-justified by recourse to evolution, which means that scientists have to be politically astute and sensitive to the uses of their work to counter these social tendencies.
Consider this: Are you just your ancestry, or can you transcend it? If that sounds like a weird question, it was actually quite important to a turn-of-the-20th-century European society in which an old hereditary aristocracy was under increasing threat from a rising middle class. And that is why the very first English textbook of Mendelian genetics concluded with the thought that “permanent progress is a question of breeding rather than of pedagogics; a matter of gametes, not of training … the creature is not made but born” (Punnett 1905, 60). Translation: Not only do we now know a bit about how heredity works, but it’s also the most important thing about you. Trust me, I’m a scientist.
Yet evolution is about how descendants come to differ from ancestors. Do we really know that your heredity, your DNA, your ancestry, is the most important thing about you? That you were born, not made? After all, we do know that you could be born into slavery or as a peasant, and come from a long line of enslaved people or peasants, and yet not have slavery or peasantry be the most important thing about you. Whatever your ancestors were may unfortunately constrain what you can become, but as a moral precept, it should not. But just as science is not purely “facts and objectivity,” ancestry is not a strictly biological concept. Human ancestry is biopolitics, not biology.
Evolution is fundamentally a theory about ancestry, and yet ancestors are, in the broad anthropological sense, sacred: ancestors are often more meaningful symbolically than biologically. Just a few years after The Origin of Species (Darwin 1859), the British politician and writer Benjamin Disraeli declared himself to be on the side of the angels, not the apes, and to “repudiate with indignation and abhorrence those new-fangled theories” (Monypenny, Flavelle, and Buckle 1920, 105). He turned his back on an ape ancestry and looked to the angel; yet, he did so as a prominent Jew-turned-Anglican, who had personally transcended his humble roots and risen to the pinnacle of the Empire. Ancestry was certainly important, and Disraeli was famously proud of his, but it was also certainly not the most important thing, not the primary determinant of his place in the world. Indeed, quite the opposite: Disraeli’s life was built on the transcendence of many centuries of Jewish poverty and oppression in Europe. Humble ancestry was there to be superseded and nobility was there to be earned; Disraeli would later become the Earl of Beaconsfield. Clearly, “are you just your ancestry” is not a value-neutral question, and “the creature is not made, but born” is not a value-neutral answer.
Ancestry being the most important thing about a person became a popular idea twice in 20th century science. First, at the beginning of the century, when the eugenics movement in America called attention to “feeble-minded stocks,” which usually referred to the poor or to immigrants (see Figure 17.4; and see Chapter 2). This movement culminated in Congress restricting the immigration of “feeble-minded races” (said to include Jews and Italians) in 1924, and the Supreme Court declaring it acceptable for states to sterilize their “feeble-minded” citizens involuntarily in 1927. After the Nazis picked up and embellished these ideas during World War II, Americans moved swiftly away from them in some contexts (e.g., for most people of European descent) while still strictly adhering in other contexts (e.g., Japanese internment camps and immigration restrictions).

Ancestry again became paramount in the drumming up of public support for the Human Genome Project in the 1990s. Public support for sequencing the human genome was encouraged by a popular science campaign that featured books titled The Book of Man (Bodmer and McKie 1997), The Human Blueprint (Shapiro 1991), and The Code of Codes (Kevles and Hood 1993). These books generally promised cures for genetic diseases and a deeper understanding of the human condition. We can certainly identify progress in molecular genetics over the last couple of decades since the human genome was sequenced, but that progress has notably not been accompanied by cures for genetic diseases, nor by deeper understandings of the human condition.
Even at the most detailed and refined levels of genetic analysis, we still don’t have much of an understanding of the actual basis by which things seem to “run in families.” While the genetic basis of simple, if tragic, genetic diseases have become well-known—such as sickle-cell anemia, cystic fibrosis, and Tay-Sachs’ Disease—we still haven’t found the ostensible genetic basis for traits that are thought to have a strong genetic component. For example, a recent genetic summary found over 12,000 genetic sites that contributed to height yet still explained only about 40-50 percent of the variation in height among European ancestry but no more than 10-20 percent of variation of other ancestries, which we know strongly runs in families (Yengo et al. 2022).
Partly in reaction to the reductionistic hype of the Human Genome Project, the study of epigenetics has become the subject of great interest. One famous natural experiment involves a Nazi-imposed famine in Holland over the winter of 1944–1945. Children born during and shortly after the famine experienced a higher incidence of certain health problems as adults, many decades later. Apparently, certain genes had been down-regulated early in development and remained that way throughout the course of life. Indeed, this modified regulation of the genes in response to the severe environmental conditions may have been passed on to their children.
Obviously one’s particular genetic constitution may play an important role in one’s life trajectory. But overvaluing that role may have important social and political consequences. In the first place, genotypes are rendered meaningful in a cultural universe. Thus, if you live in a strongly patriarchal society and are born without a Y chromosome (since human males are chromosomally XY and females XX), your genotype will indeed have a strong effect upon your life course. So even though the variation is natural, the consequences are political. The mediating factors are the cultural ideas about how people of different sexes ought to be treated, and the role of the state in permitting certain people to develop and thrive. More broadly, there are implications for public education if variation in intelligence is genetic. There are implications for the legal system if criminality is genetic. There are implications for the justice system if sexual preference, or sexual identity, is genetic. There are implications for the development of sports talent if that is genetic. And yet, even for the human traits that are more straightforward to measure and known to be strongly heritable, the DNA base sequence variation seems to explain little.
Genetic determinism or hereditarianism is the idea that “the creature is made, not born”—or, in a more recent formulation by James Watson, that “our fate is in our genes.” One of the major implications drawn from genetic determinism is that the feature in question must inevitably express itself; therefore, we can’t do anything about it. Therefore, we might as well not fund the social programs designed to ameliorate economic inequality and improve people’s lives, because their courses are fated genetically. And therefore, they don’t deserve better lives.
All of the “therefores” in the preceding paragraph are open to debate. What is important is that the argument relies on a very narrow understanding of the role of genetics in human life, and it misdirects the causes of inequality from cultural to natural processes. By contrast, instead of focusing on genes and imagining them to place an invisible limit upon social progress, we can study the ways in which your DNA sequence does not limit your capability for self-improvement or fix your place in a social hierarchy. In general, two such avenues exist. First, we can examine the ways in which the human body responds and reacts to environmental variation: human adaptability and plasticity. This line of research began with the anthropometric studies of immigrants by Franz Boas in the early 20th century and has now expanded to incorporate the epigenetic inheritance of modified human DNA. And second, we can consider how human lives are shaped by social histories—especially the structural inequalities within the societies in which they grow up.
Although it arises and is refuted every generation, the radical hereditarian position (genetic determinism) perennially claims to speak for both science and evolution. It does not. It is the voice of a radical fringe—perhaps naive, perhaps evil. It is not the authentic voice of science or of evolution. Indeed, keeping Charles Darwin’s name unsullied by protecting it from association with bad science often seems like a full-time job. Culture and epigenetics are very much a part of the human condition, and their roles are significant parts of the complete story of human evolution.
Adaptationism and the Panglossian Paradigm
The story of human evolution, and the evolution of all life for that matter, is never settled because evolution is ongoing. Additionally, because the conditions that shape evolutionary trajectories are not predetermined, evolution itself is emergent. Even during periods of ecological stability, when fewer macroevolutionary changes occur, populations of organisms continue to experience change. When ecological stability is disrupted, populations must adapt to the changes. Darwin explained in naturalistic terms how animals adapt to their environments: traits that contribute to an organism’s ability to survive and reproduce in specific environments will become more common. The most “fit”—those organisms best suited to the current environmental conditions in which they live—have survived over eons of the history of life on earth to cocreate ecosystems full of animals and plants. Our own bodies are full of evident adaptations: eyes for seeing, ears for hearing, feet for walking on, and so forth.
But what about hands? Feet are adapted to be primarily weight-bearing structures (rather than grasping structures, as in the apes) and that is what we primarily use them for. But we use our hands in many ways: for fine-scale manipulation, greeting, pointing, stimulating a sexual partner, writing, throwing, and cooking, among other uses. So which of these uses express what hands are “for,” when all of them express what hands do?
Gould and Lewontin (1979) illustrate the problem with assuming that the function of a trait defines its evolutionary cause. Consider the case of Dr. Pangloss—the protagonistic of Voltaire’s Candide—who believed that we lived in the best of all possible worlds. Gould and Lewontin use his pronouncement that “noses were made for spectacles and so we have spectacles” to demonstrate the problem with assuming any trait has evolved for a specific purpose. Identifying a function of a trait does not necessitate that the function is the ultimate cause of the trait. Individual traits are not under selection pressures in isolation; in fact, an entire organism must be able to survive and reproduce in their environment. When natural selection results in adaptations, changes that occur in some traits can have cascading effects throughout the phenotype and features that are not under selection pressure can also change.
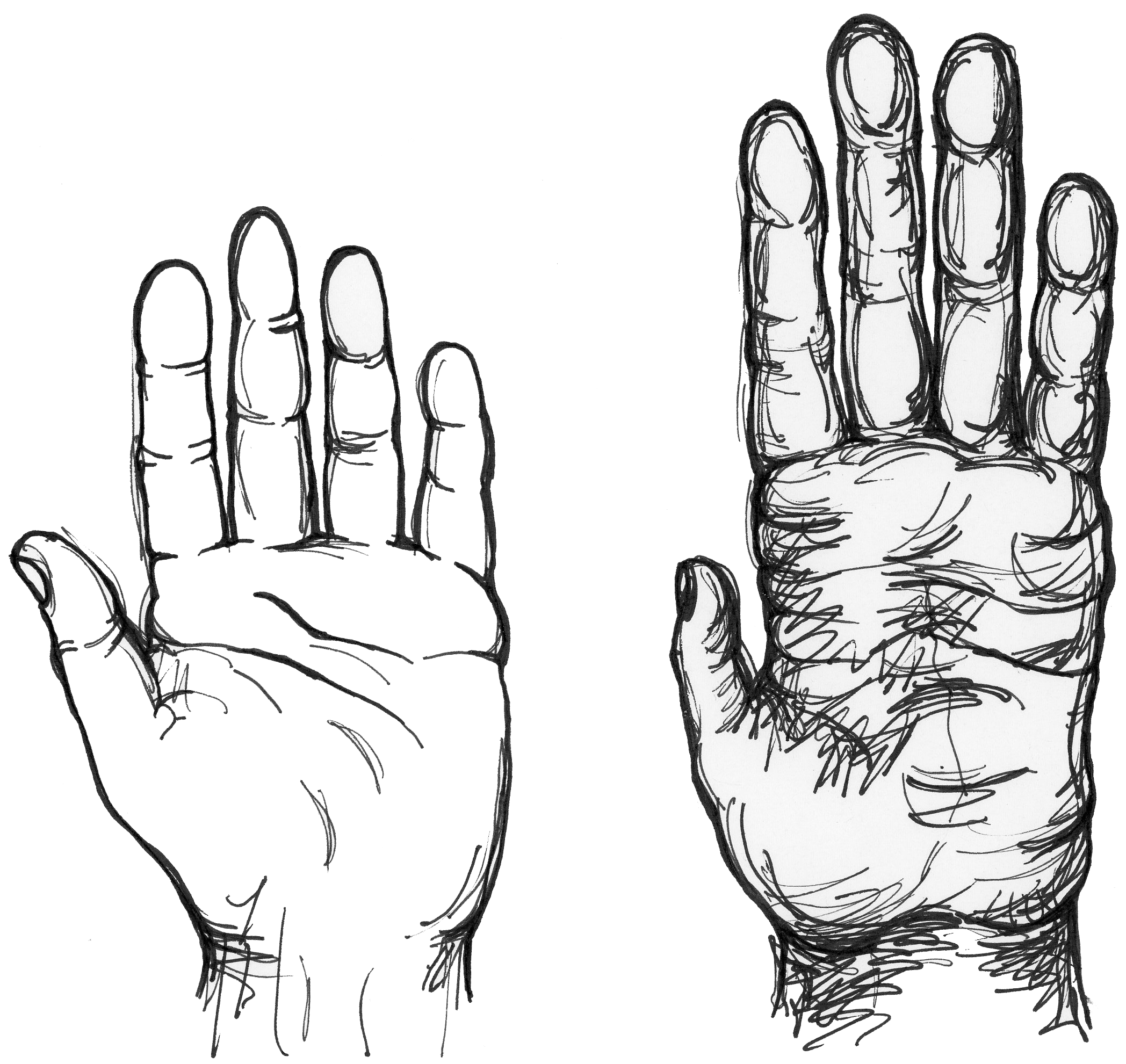
There is an important lesson in recognizing that what things do in the present is not a good guide to understanding why they came to exist. Gunpowder was invented for entertainment—only later was it adopted for killing people. The Internet was invented to decentralize computers in case of a nuclear attack—and only later adopted for social media. Apes have short thumbs and use their hands in locomotion; our ancestors stopped using their hands in locomotion by about six million years ago and had fairly modern-looking hands by about two million years ago. We can speculate that a combination of selection for abstract thought and dexterity led to evolution of the human hand, with its capability for toolmaking that exceeds what apes can do (see Figure 17.5). But let’s face it—how many tools have you made today?
Consequently, we are obliged to see the human foot as having a purpose to which it is adapted and the human hand as having multiple purposes, most of which are different from what it originally evolved for. Paleontologists Gould and Elisabeth Vrba suggested that an original use be regarded as an adaptation and any additional uses be called “exaptations.” Thus, we would consider the human hand to be an adaptation for toolmaking and an exaptation for writing. So how do we know whether any particular feature is an adaptation, like the walking foot, rather than an exaptation, like the writing hand? Or more broadly, how can we reason rigorously from what a feature does to what it evolved for?
The answer to the question “what did this feature evolve for?” creates an origin myth. This origin myth contains three assumptions: (1) features can be isolated as evolutionary units; (2) there is a specific reason for the existence of any particular feature; and (3) there is a clear and simplistic explanation for why the feature evolved.
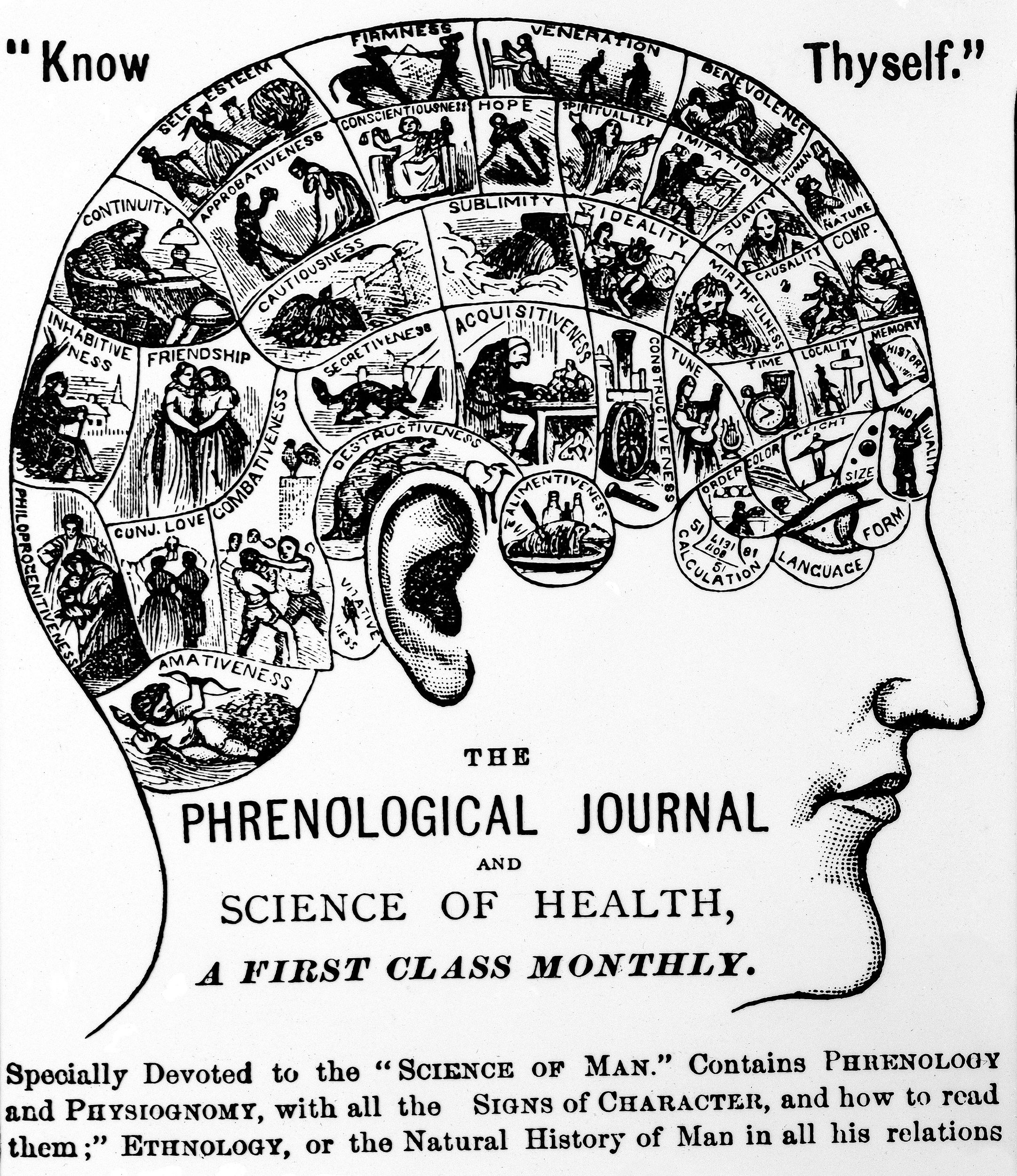
The first assumption was appreciated a century ago as the “unit-character problem.” Are the units by which the body grows and evolves the same as units we name? This is clearly not the case: we have genes and we have noses, and we have genes that affect noses, but we don’t have “nose genes.” What is the relationship between the evolving elements that we see, identify, and name, and the elements that biologically exist and evolve? It is hard to know, but we can use the history of science as a guide to see how that fallacy has been used by earlier generations. Back in the 19th century, the early anatomists argued that since the brain contained the mind, they could map different mental states (acquisitiveness, punctuality, sensitivity) onto parts of the brain. Someone who was very introspective, say, would have an enlarged introspection part of the brain, a cranial bulge to represent the hyperactivity of this mental state. The anatomical science was known as phrenology, and it was predicated on the false assumption that units of thought or personality or behavior could be mapped to distinct parts of the brain and physically observed (see Figure17.6). This is the fallacy of reification, imagining that something named is something real.
Long alt text: Side view of human head. At the top are the words “Know Thyself.” On the upper head are small illustrations and word qualities such as “friendship,” “self-esteem,” and “secretiveness.” On the lower part of the man’s man’s face are the words The Phrenological Journal and Science of Health, A First Class Monthly. The caption at the bottom reads: “Specially devoted to the ‘.’ Contains PHRENOLOGY and PHYSIOGNOMY, with all the SIGNS OF CHARACTER, and how to read them; ETHNOLOGY, or the Natural History of Man in all his relations.” (All emphases in original.)
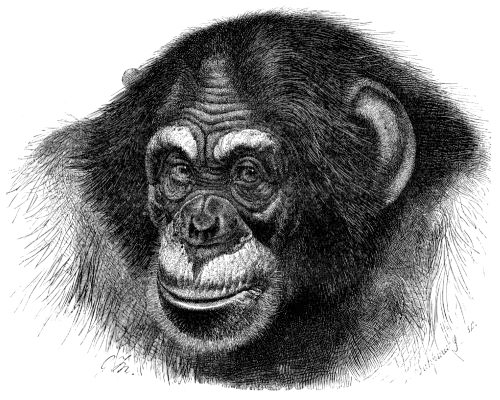
The second assumption, that everything has a reason, has long been recognized as a core belief of religion. Our desire to impose order and simplicity on the workings of the universe, however, does not constrain it to obey simple and orderly causes. Magic, witchcraft, spirits, and divine agency are all powerful explanations for why things happen. Consequently, it is probably not a good idea to lump natural selection in with those. Sometimes things do happen for a reason, of course, but other times things happen as byproducts of other things, or for very complicated and entangled reasons, or for no reason at all. What phenomena have reasons and thereby merit explanation? Chimpanzees have very large testicles, and we think we know why: their promiscuous sexual behavior triggers intense competition for high sperm count. But chimpanzees also have very large ears, but much less scientific attention has been paid to this trait (see Figure 17.7). Why not? Why should there be a reason for chimp testicles but not for chimp ears? What determines the kinds of features that we try to explain, as opposed to the ones that we do not? Again, the assumption that any specific feature has a reason is metaphysical; that is to say, it may be true in any particular case, but to assume it in all cases is gratuitous.
And third, the possibility of knowing what the reason for any particular feature is, assuming that it has one, is a challenge for evolutionary epistemology (the theory of how we know things). Consider the big adaptations of our lineage: bipedalism and language. Nobody doubts that they are good, and they evolved by natural selection, and we know how they work. But why did they evolve? If talking and walking are simply better than not talking and not walking, then why did they evolve in just a single branch of the ape lineage in the primate family tree? We don’t know what bipedalism evolved for, although there are plenty of speculations: walking long distances, running long distances, cooling the head, seeing over tall grass, carrying babies, carrying food, wading, threatening, counting calories, sexual display, and so on. Neither do we know what language evolved for, although there are speculations: coordinating hunting, gossiping, manipulating others. But it is also possible that bipedality is simply the way that a small arboreal ape travels on the ground, if it isn’t in the treetops. Or that language is simply the way that a primate with small canine teeth and certain mental propensities comes to communicate. If that were true, then there might be no reason for bipedality or language: having the unique suite of preconditions and a fortuitous set of circumstances simply set them in motion, and natural selection elaborated and explored their potentials. It is possible that walking and talking simply solved problems that no other lineage had ever solved; but even if so, the fact remains that the rest of the species in the history of life have done pretty well without having solved them.
It is certainly very optimistic to think that all three assumptions (that organisms can be meaningfully atomized, that everything has a reason, and that we can know the reason) would be simultaneously in effect. Indeed, just as there are many ways of adapting (genetically, epigenetically, behaviorally, culturally), there are also many ways of being nonadaptive, which would imply that there is no reason at all for the feature in question.
First, there is the element of randomness of population histories. There are more cases of sickle-cell anemia among sub-Saharan Africans than other peoples, and there is a reason for it: carriers of sickle-cell anemia have a resistance to malaria, which is more frequent in parts of Africa (as discussed in Chapters 4 and 14). But there are more cases of a blood disease called variegated porphyria, a rare genetic metabolic disorder, in the Afrikaners of South Africa (descendants of mostly Dutch settlers in the 17th century) than in other peoples, and there is no reason for it. Yet we know the cause: One of the founding Dutch colonial settlers had the allele–a variant of a gene–and everyone in South Africa with it today is her descendant. But that is not a reason—that is simply an accident of history.
Second, there is the potential mismatch between the past and the present. The value of a particular feature in the past may be changed as the environmental circumstances change. Our species is diurnal, and our ancestors were diurnal. But beginning around a few hundred thousand years ago, our ancestors could build fires, which extended the light period, which was subsequently further amplified by lamps and candles. And over the course of the 20th century, electrical power has made it possible for people to stay up very late when it is dark—working, partying, worrying—to a greater extent than any other closely related species. In other words, we evolved to be diurnal, yet we are now far more nocturnal than any of our recent ancestors or close relatives. Are we adapting to nocturnality? If so, why? Does it even make any sense to speak of the human occupation of a nocturnal ape niche, despite the fact that we empirically seem to be doing just that? And if so, does it make sense to ask what the reason for it is?
Third, there is a genetic phenomenon known as a selective sweep, or the hitchhiker effect. Imagine three genes—A, B, and C—located very closely together on a chromosome. They each have several variants, or alleles, in the population. Now, for whatever reason, it becomes beneficial to have one of the B alleles, say B4; this B4 allele is now under strong positive selection. Obviously, we will expect future generations to be characterized by mostly B4. But what was B4 attached to? Because whatever A and C alleles were adjacent to it will also be quickly spread, simply by virtue of the selection for B4. Even if the A and C alleles are not very good, they will spread because of the good B4 allele between them. Eventually the linkage groups will break up because of genetic crossing-over in future generations. But in the meantime, some random version of genes A and C are proliferating in the species simply because they are joined to superior allele B4. And clearly, the A and C alleles are there because of selection—but not because of selection for them!
Fourth, some features are simply consequences of other properties rather than adaptations to external conditions. We already noted the phenomenon of allometric growth, in which some physical features have to outgrow others to maintain function at an increased size. Can we ask the reason for the massive brow ridges of Homo erectus, or are brow ridges simply what you get when you have a conjunction of thick skull bones, a large face, and a sloping forehead—and, thus, again would have a cause but no reason?
Fifth, some features may be underutilized and on the way out. What is the reason for our two outer toes? They aren’t propulsive, they don’t do anything, and sometimes they’re just in the way. Obviously they are there because we are descended from ancestors with five digits on their hands and feet. Is it possible that a million years from now, we will just have our three largest toes, just as the ancestors of the horse lost their digits in favor of a single hoof per limb? Or will our outer toes find another use, such as stabilizing the landings in our personal jet-packs? For the time being, we can just recognize vestigiality as another nonadaptive explanation for the presence of a given feature.
Finally, Darwin himself recognized that many obvious features do not help an animal survive. Some things may instead help an animal breed. The peacock’s tail feathers do not help it eat, but they do help it mate. There is competition, but only against half of the species. Darwin called this sexual selection. Its result is not a fit to the environment but, rather, a fit to the opposite sex. In some species, that is literally the case, as the male and female genitalia have specific ways of anatomically fitting together. The specific form is less important than the specific match, so inquiring about the reason for a particular form of the reproductive anatomy may be misleading. The specific form may be effectively random, as long as it fits the opposite sex and is different from the anatomies of other species. Nor is sexual selection the only form of selection that can affect the body differently from natural selection. Competition might also take place between biological units other than organisms—perhaps genes, perhaps cells, or populations, or species. The spread of cultural things, such as head-binding or cheap refined fructose or forced labor, can have significant effects upon bodies, which are also not adaptations produced by natural selection. They are often adaptive physiological responses to stresses but not the products of natural selection.
With so many paths available by which a physical feature might have organically arisen without having been the object of natural selection, it is unwise to assume that any individual trait is an adaptation. And that generalization applies to the best-known, best-studied, and most materially based evolutionary adaptations of our lineage. But our cultural behaviors are also highly adaptive, so what about our most familiar social behaviors? Patriarchy, hierarchy, warfare—are these adaptations? Do they have reasons? Are they good for something?
This is where some sloppy thinking has been troublesome. What would it mean to say that patriarchy evolved by natural selection in the human species? If, on the one hand, it means that the human mind evolved by natural selection to be able to create and survive in many different kinds of social and political regimes, of which patriarchy is one, then biological anthropologists will readily agree. If, on the other hand, it means that patriarchy evolved by natural selection, that implies that patriarchy is genetically determined (since natural selection is a genetic process) and out-reproduced the alleles for other, more egalitarian, social forms. This in turn would imply that patriarchy is an adaptation and therefore of some beneficial value in the past and has become an ingrained part of human nature today. This would be bad news, say, if you harbored ambitions of dismantling it. Dismantling patriarchy in that case would be to go against nature, a futile gesture. In other words, this latter interpretation would be a naturalistic manifesto for a conservative political platform: don’t try to dismantle the patriarchy, because it is within us, the product of evolution—suck it up and live with it.
Here, evolution is being used as a political instrument for transforming the human genome into an imaginary glass ceiling against equality. There is thus a convergence between the pseudo-biology of crude adaptationism (the idea that everything is the product of natural selection) and the pseudo-biology of hereditarianism. Naturalizing inequality is not the business of evolutionary theory, and it represents a difficult moral position for a scientist to adopt, as well as a poor scientific position.
Concluding Thoughts
Now that you have finished reading this chapter, you are equipped to understand the historical and political dimensions of evolution. Evolution is an ongoing process of change and diversification. Evolutionary theory is a tool that we use to understand this process. The development of evolutionary theory is shaped both by scientific innovation and political engagement. Since Darwin first articulated natural selection as an observable mechanism by which species adapt to their environments, our understanding of evolution has grown. Initially, scientists focused on the adaptive aspects of evolution. However, with the emergence of genetics, our understanding of heredity and the level at which evolution acts has changed. Genetics led to a focus on the molecular dimensions of evolution. For some, this focus resulted in reductive accounts of evolution. Further developments in our understanding of evolution shifted our view to epigenetic processes and how organisms shape their own evolutionary pressures (e.g., niche construction).
Evolutionary theory will continue to develop in the future as we invent new technologies, describe new dimensions of biology, and experience cultural changes. Current innovations in evolutionary theory are asking us to consider evolutionary forces beyond natural selection and genetics to include the ways organisms shape their environments (niche construction), inheritances beyond genetics (inclusive inheritance), constraints on evolutionary change (developmental bias), and the ability of bodies to change in response to external factors (plasticity). The future of evolutionary theory looks bright as we continue to explore these and other dimensions. Biological anthropology is well-positioned to be a lively part of this conversation, as it extends standard evolutionary theory by considering the role of culture, social learning, and human intentionality in shaping the evolutionary trajectories of humans (Zeder 2018). Remember, at root, human evolutionary theory consists of two propositions: (1) the human species is descended from other similar species and (2) natural selection has been the primary agent of biological adaptation. Pretty much everything else is subject to some degree of contestation.
Review Questions
- How is the study of your ancestors biopolitical, not just biological? Does that make it less scientific or differently scientific?
- What was gained by reducing organisms to genotypes and species to gene pools? What is gained by reintroducing bodies and species into evolutionary studies?
- How do genetic or molecular studies complement anatomical studies of evolution?
- How are you reducible to your ancestry? If you could meet your ancestors from the year 1700 (and you would have well over a thousand of them!), would their lives be meaningfully similar to yours? Would you even be able to communicate with them?
- The molecular biologist François Jacob argued that evolution is more like a tinkerer than an engineer. In what ways do we seem like precisely engineered machinery, and in what ways do we seem like jerry-rigged or improvised contraptions?
- How might biological anthropology contribute to future developments in evolutionary theory?
Key Terms
Adaptation: A fit between the organism and environment.
Adaptationism: The idea that everything is the product of natural selection.
Allele: A genetic variant.
Allometry: The differential growth of body parts.
Canalization: The tendency of a growing organism to be buffered toward normal development.
Epigenetics: The study of how genetically identical cells and organisms (with the same DNA base sequence) can nevertheless differ in stably inherited ways.
Eugenics: An idea that was popular in the 1920s that society should be improved by breeding “better” kinds of people.
Evo-devo: The study of the origin of form; a contraction of “evolutionary developmental biology.”
Exaptation: An additional beneficial use for a biological feature.
Extinction: The loss of a species from the face of the earth.
Gene: A stretch of DNA with an identifiable function (sometimes broadened to include any DNA with recognizable structural features as well).
Gene pool: Hypothetical summation of the entire genetic composition of population or species.
Genotype: Genetic constitution of an individual organism.
Hereditarianism: The idea that genes or ancestry is the most crucial or salient element in a human life. Generally associated with an argument for natural inequality on pseudo-genetic grounds.
Hox genes: A group of related genes that control for the body plan of an embryo along the head-tail axis.
Inheritance of acquired characteristics: The idea that you pass on the features that developed during your lifetime, not just your genes; also known as Lamarckian inheritance.
Natural selection: A consistent bias in survival and fertility, leading to the overrepresentation of certain features in future generations and an improved fit between an average member of the population and the environment.
Niche construction: The active engagement by which species transform their surroundings in favorable ways, rather than just passively inhabiting them.
Phenotype: Observable manifestation of a genetic constitution, expressed in a particular set of circumstances. The suite of traits of an organism.
Phrenology: The 19th-century anatomical study of bumps on the head as an indication of personality and mental abilities.
Plasticity: The tendency of a growing organism to react developmentally to its particular conditions of life.
Punctuated equilibria: The idea that species are stable through time and are formed very rapidly relative to their duration. (The opposite theory, that species are unstable and constantly changing through time, is called phyletic gradualism.)
Scientific racism: The use of pseudoscientific evidence to support or legitimize racial hierarchy and inequality.
Sexual selection: Natural selection arising through preference by one sex for certain characteristics in individuals of the other sex.
Species selection: A postulated evolutionary process in which selection acts on an entire species population, rather than individuals.
About the Authors

Jonathan Marks, Ph.D.
University of North Carolina at Charlotte, jmarks@uncc.edu
Jonathan Marks is Professor of Anthropology at the University of North Carolina at Charlotte. He has published many books and articles on broad aspects of biological anthropology. In 2006 he was elected a Fellow of the American Association for the Advancement of Science. In 2012 he was awarded the First Citizen’s Bank Scholar’s Medal from UNC Charlotte. In recent years he has been a Visiting Research Fellow at the ESRC Genomics Forum in Edinburgh, a Visiting Research Fellow at the Max Planck Institute for the History of Science in Berlin, and a Templeton Fellow at the Institute for Advanced Study at Notre Dame. His work has received the W. W. Howells Book Prize and the General Anthropology Division Prize for Exemplary Cross-Field Scholarship from the American Anthropological Association as well as the J. I. Staley Prize from the School for Advanced Research. Two of his books are titled What It Means to Be 98% Chimpanzee and Why I Am Not a Scientist, but actually he is about 98 percent scientist and not a chimpanzee.

Adam P. Johnson, M.A.
University of North Carolina at Charlotte/University of Texas at San Antonio, ajohn344@uncc.edu
Adam Johnson is a doctoral candidate at the University of Texas at San Antonio and part-time lecturer at the University of North Carolina at Charlotte. He earned his M.A. in anthropology at UNC-Charlotte in 2017 and will complete his Ph.D. in anthropology at UTSA by 2024. His interests include human-animal relations, science studies, primate behavior, ecology, and the history of anthropology. His recent research project analyzes the social, historical, political, and evolutionary dimensions that shape human-javelina encounters. His goal is to understand how humans and animals find ways to get along in a precarious world.
For Further Exploration
Ackermann, Rebecca Rogers, Alex Mackay, and Michael L. Arnold. 2016. “The Hybrid Origin of ‘Modern’ Humans.” Evolutionary Biology 43 (1): 1–11.
Bateson, Patrick, and Peter Gluckman. 2011. Plasticity, Robustness, Development and Evolution. New York: Cambridge University Press.
Cosans, Christopher E. 2009. Owen’s Ape and Darwin’s Bulldog: Beyond Darwinism and Creationism. Bloomington, IN: Indiana University Press.
Desmond, Adrian, and James Moore. 2009. Darwin’s Sacred Cause: How a Hatred of Slavery Shaped Darwin’s Views on Human Evolution. New York: Houghton Mifflin Harcourt.
Dobzhansky, Theodosius, Francisco J. Ayala, G. Ledyard Stebbins, and James W. Valentine. 1977. Evolution. San Francisco: W.H. Freeman and Company.
Fuentes, Agustín. 2017. The Creative Spark: How Imagination Made Humans Exceptional. New York: Dutton.
Gould, Stephen J. 2003. The Structure of Evolutionary Theory. Cambridge, MA: Harvard University Press.
Haraway, Donna J. 1989. Primate Visions: Gender, Race, and Nature in the World of Modern Science. New York: Routledge.
Huxley, Thomas. 1863. Evidence as to Man’s Place in Nature. London: Williams & Norgate.
Jablonka, Eva, and Marion J. Lamb. 2005. Evolution in Four Dimensions: Genetic, Epigenetic, Behavioral, and Symbolic Variation in the History of Life. Cambridge, MA: The MIT Press.
Kuklick, Henrika, ed. 2008. A New History of Anthropology. New York: Blackwell.
Laland, Kevin N., Tobias Uller, Marcus W. Feldman, Kim Sterelny, Gerd B. Muller, Armin Moczek, Eva Jablonka, and John Odling-Smee. 2015. “The Extended Evolutionary Synthesis: Its Structure, Assumptions and Predictions.” Proceedings of the Royal Society, Series B 282 (1813): 20151019.
Lamarck, Jean Baptiste. 1809. Philosophie Zoologique. Paris: Dentu.
Landau, Misia. 1991. Narratives of Human Evolution. New Haven: Yale University Press.
Lee, Sang-Hee. 2017. Close Encounters with Humankind: A Paleoanthropologist Investigates Our Evolving Species. New York: W. W. Norton.
Livingstone, David N. 2008. Adam’s Ancestors: Race, Religion, and the Politics of Human Origins. Baltimore: Johns Hopkins University Press.
Marks, Jonathan. 2015. Tales of the Ex-Apes: How We Think about Human Evolution. Berkeley, CA: University of California Press.
Pigliucci, Massimo. 2009. “The Year in Evolutionary Biology 2009: An Extended Synthesis for Evolutionary Biology.” Annals of the New York Academy of Sciences 1168: 218–228.
Simpson, George Gaylord. 1949. The Meaning of Evolution: A Study of the History of Life and of Its Significance for Man. New Haven: Yale University Press.
Sommer, Marianne. 2016. History Within: The Science, Culture, and Politics of Bones, Organisms, and Molecules. Chicago: University of Chicago Press.
Stoczkowski, Wiktor. 2002. Explaining Human Origins: Myth, Imagination and Conjecture. New York: Cambridge University Press.
Tattersall, Ian, and Rob DeSalle. 2019. The Accidental Homo sapiens: Genetics, Behavior, and Free Will. New York: Pegasus.
References
Barton, Robert A. 1996. “Neocortex Size and Behavioural Ecology in Primates.” Proceedings of the Royal Society of London. Series B: Biological Sciences 263 (1367): 173–177.
Bodmer, Walter, and Robin McKie. 1997. The Book of Man: The Hman Genome Project and the Quest to Discover our Genetic Heritage. Oxford University Press.
Darwin, Charles. 1859. On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life. London: J. Murray.
Darwin, Charles. 1871. The Descent of Man, and Selection in Relation to Sex. London: J. Murray.
Dawkins, Richard. 1976. The Selfish Gene. Oxford University Press.
Deacon, T. W. 1998. The Symbolic Species: The Co-evolution of Language and the Brain. W. W. Norton & Company.
Eldredge, N., and S. J. Gould. 1972. “Punctuated Equilibria: An Alternative to Phyletic Gradualism.” In Models in Paleobiology, edited by T. J. Schopf, 82–115. San Francisco: W. H. Freeman.
Gould, Stephen J. 2003. The Structure of Evolutionary Theory. Cambridge, MA: Harvard University Press.
Gould, Stephen J. 1996. Mismeasure of Man. New York: WW Norton & Company.
Gould, Stephen Jay, and Richard C. Lewontin. 1979. “The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme.” Proceedings of the Royal Society of London. Series B: Biological Sciences 205 (1151): 581–598.
Haeckel, Ernst. 1868. Natürliche Schöpfungsgeschichte. Berlin: Reimer.
Huxley, Thomas Henry. 1863. Evidence as to Man’s Place in Nature. London: Williams and Norgate.
Kaufman, Thomas C., Mark A. Seeger, and Gary Olsen. 1990. “Molecular and Genetic Organization of the Antennapedia Gene Complex of Drosophila melanogaster.” Advances in Genetics 27: 309–362.
Kellogg, Vernon. 1917. Headquarters Nights. Boston: The Atlantic Monthly Press.
Kevles, Daniel J., and Leroy Hood. 1993. The Code of Codes: Scientific and Social Issues in the Human Genome Project. Cambridge, MA: Harvard University Press.
Lewontin, Richard, Steven Rose, and Leon Kamin. 2017. Not in Our Genes : Biology, Ideology, and Human Nature, 2nd ed. Chicago: Haymarket Books.
Lloyd, Elisabeth A., and Stephen J. Gould. 1993. “Species Selection on Variability.” Proceedings of the National Academy of Sciences 90 (2): 595–599.
Marks, Jonathan. 2015. “The Biological Myth of Human Evolution.” In Biologising the Social Sciences: Challenging Darwinian and Neuroscience Explanations, edited by David Canter and David A. Turner, 59–78. London: Routledge.
Monypenny, William Flavelle, and George Earle Buckle. 1929. The Life of Benjamin Disraeli, Earl of Beaconsfield, Volume II: 1860–1881. London: John Murray.
Potts, Rick. 1998. “Variability Selection in Hominid Evolution.” Evolutionary Anthropology 7: 81–96.
Punnett, R. C. 1905. Mendelism. Cambridge: Macmillan and Bowes.
Shapiro, Robert. 1991. The Human Blueprint: The Race to Unlock the Secrets of Our Genetic Script. New York: St. Martin’s Press.
Shultz, Susanne, Emma Nelson, and Robin Dunbar. 2012. “Hominin Cognitive Evolution: Identifying Patterns and Processes in the Fossil and Archaeological Record.” Philosophical Transactions of the Royal Society B: Biological Sciences 367 (1599): 2130–2140.
Spencer, Herbert. 1864. Principles of Biology. London: Williams and Norgate.
Watson, James D. 1990. “The Human Genome Project: Past, Present, and Future.” Science 248 (4951): 44–49.
Yengo, L., Vedantam, S., Marouli, E., Sidorenko, J., Bartell, E., Sakaue, S., Graff, M., Eliasen, A.U., Jiang, Y., Raghavan, S. and Miao, J., 2022. A saturated map of common genetic variants associated with human height. Nature, 610 (7933): 704-712.
Zeder, Melinda A. 2018. “Why Evolutionary Biology Needs Anthropology: Evaluating Core Assumptions of the Extended Evolutionary Synthesis.” Evolutionary Anthropology: Issues, News, and Reviews 27 (6): 267–284.
A sequence of DNA that provides coding information for the construction of proteins.
Genetic constitution of an individual organism.
Hypothetical summation of the entire genetic composition of population or species.
The loss of a species from the face of the earth.
A postulated evolutionary process in which selection acts on an entire species population, rather than individuals.
The idea that species are stable through time and are formed very rapidly relative to their duration. (The opposite theory, that species are unstable and constantly changing through time, is called phyletic gradualism.)
A fit between the organism and environment.
The study of the origin of form; a contraction of “evolutionary developmental biology.”
A group of related genes that control for the body plan of an embryo along the head-tail axis.
The differential growth of body parts.
A set of outwardly observable characteristics for an individual.
The idea that you pass on the features that developed during your lifetime, not just your genes; also known as Lamarckian inheritance.
The tendency of a growing organism to react developmentally to its particular conditions of life.
The tendency of a growing organism to be buffered toward normal development.
The study of how genetically identical cells and organisms (with the same DNA base sequence) can nevertheless differ in stably inherited ways.
The active engagement by which species transform their surroundings in favorable ways, rather than just passively inhabiting them.
A consistent bias in survival and fertility, leading to the overrepresentation of certain features in future generations and an improved fit between an average member of the population and the environment.
The use of pseudoscientific evidence to support or legitimize racial hierarchy and inequality.
A set of beliefs and practices that involves the controlled selective breeding of human populations with the hope of improving their heritable qualities, especially through surgical procedures like sterilization and legal rulings that affect marriage rights for interracial couples.
The idea that genes or ancestry is the most crucial or salient element in a human life. Generally associated with an argument for natural inequality on pseudo-genetic grounds.
An additional beneficial use for a biological feature.
The 19th-century anatomical study of bumps on the head as an indication of personality and mental abilities.
A genetic variant.
The selection for traits that increase mating success. This occurs via intersexual selection and intrasexual selection.
The idea that everything is the product of natural selection.
