4 Motor Output
Motoki Sato, MS
My name is Motoki Sato and I’m a Masters student in Kinesiology. My area of focus and interest is exercise science, so you may notice my chapter being written through the lens of physiology. This chapter will focus on motor output and some of the key players involved in the mechanisms of both sensory input and voluntary and involuntary movement. I picked the topics that were of most interest to me! I will just be scratching the surface and if you would like to dig deeper, take a look at the articles cited in the chapter. I hope you enjoy!
Introduction
Motor output is defined as “impulses conducted by the brain and spinal cord to the muscles and glands“. Similarly, motor control is defined as “the process of initiating, directing, and grading purposeful voluntary movement ” (Medical Dictionary for the Health Professions and Nursing, 2012). It involves a cascade of information through different channels and segments through sensory input (Knierham, 2020), the neuromuscular system, and the skeletal system (Nishikawa et al., 2007). Through receptors, we can perceive the world through various sensations and determine our movement based on the information (Gadhvi & Waseem, 2022). Specifically in terms of motor control, the mechanoreceptors are responsible for sensing mechanical stimuli and through the Central Nervous System (CNS), control the movement of muscles. Mechanoreception combined with other sensory receptors provide crucial information, but otherwise meaningless unless we’re able to react upon it. Sensory input from one’s environments such as running away from danger or getting up from the coach to grab something to eat when you’re hungry is part of the motor control system. Although this concept seems rather simple and straightforward, there are many components other than sensory input that contribute to conscious actions. In all instances, the end result is a series of instructions to specific muscles within the body, instructing them to apply pressure against external objects or opposing forces.
https://www.getbodysmart.com/motor-system/pyramidal-tract-pathway/
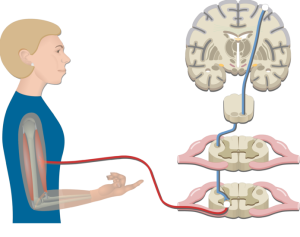
The Central Nervous System
The central nervous system (CNS) plays a critical role in generating and controlling motor output. The CNS includes the brain and spinal cord, which work together to process and integrate sensory information and generate appropriate motor commands.
https://romancechem4.blogspot.com/2021/05/human-central-nervous-system-diagram.html
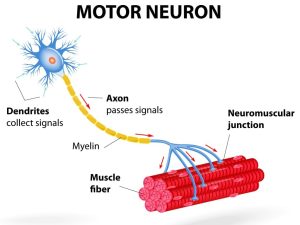
Motor Neuron
A motor neuron is a type of nerve cell that is responsible for controlling voluntary and involuntary muscle movement (Zayia & Tadi, 2023). There are two types of motor neurons, the upper and lower motor neurons. The upper motor neurons start in the cerebral cortex and travel down the brainstem or spinal cord, while the lower motor neurons start at the spinal cord and branch out to the muscles and glands of the body (Zayia & Tadi, 2023). This chapter will be focusing mainly on the lower motor neurons. Lower motor neurons instruct the muscles to contract and generate movement in response to various stimuli, such as touch, sound, or visual cues. There are two main types of motor neurons – somatic motor neurons and autonomic motor neurons. Somatic motor neurons control the contraction of skeletal muscles, while autonomic motor neurons control the activity of glands, smooth muscle, and cardiac muscle. Motor neurons are crucial for maintaining proper motor function and coordination and are solely responsible for communication with muscles, thus every movement is dependent on the motor neuron.
https://alstreatment.com/motor-neuron-disease-als-difference/Alpha Motor Neuron
Alpha Motor Neuron
Alpha motor neurons, innervate and stimulate skeletal muscle to produce contractions, which result in movement (Goyal & Chad, 2014). The cell body of an alpha motor neuron is located in the anterior horn of the spinal cord, and its axon extends out of the spinal cord and connects to the muscle fibers it controls (Zayia & Tadi, 2023). Alpha motor neurons are also the most prevalent population of motor neurons located in the spinal cord (Goyal & Chad, 2014). Each alpha motor neuron typically innervates multiple extrafusal muscle fibers (muscle fibers that move joints and are located outside of the muscle spindles), forming a motor unit (Goyal & Chad, 2014). Furthermore, each axon of the alpha motor neuron can branch into only a small number of muscle fibers of smaller muscles responsible for fine and precisely controlled movements (Webb, 2017). Examples of the muscles responsible for precise and delicate movements include extraocular muscles, the larynx, and the intrinsic muscles of the hand (Hammerstad, 2007). On the other hand, an axon of the alpha motor neuron may branch into several hundred fibers of larger muscles responsible for strong, gross movements. Examples of the muscles responsible for forceful movements that demand less precise control include the back and thigh muscles. Alpha motor neurons play a crucial role in initiating muscle contractions and movement both fine and gross, by innervating and stimulating skeletal muscle fibers.
Gamma Motor Neuron
Gamma motor neurons or fusimotor neurons are a type of motor neuron that are involved in regulating the contraction of intrafusal muscle fibers, which are found within a specialized sensory organ called the muscle spindle (more detail in the muscle spindles section) (Goyal & Chad, 2014). Additionally, gamma motor neurons make up about one third of the lower motor neurons located in the spinal cord. Unlike alpha motor neurons, stimulation of gamma motor neurons is unable to cause movement of a joint, however, it exerts tension on the intrafusal fibers (Michael-Titus et al., 2010). Since gamma motor neurons innervate intrafusal muscle fibers, it’s responsible for regulating the sensitivity of the muscle spindle by adjusting the tension of the fibers (Hammerstad, 2007). When gamma motor neurons are activated, the intrafusal muscle fibers are contracted, which allows the muscle spindle to detect even slight changes in muscle length, and provides more accurate feedback to the central nervous system, which improves motor control.
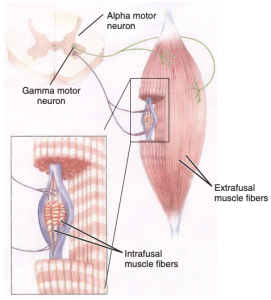
Alpha Gamma Coactivation
Alpha-gamma coactivation or the stretch reflex occurs when the alpha and gamma motor neurons are stimulated simultaneously (Webb, 2017). Remember, alpha motor neurons are in charge of the contraction of extrafusal muscle fibers, while gamma motor neurons regulate the contraction of intrafusal muscle fibers. The activation of both these motor neurons is necessary for the proper functioning of the muscle spindle. As the ends of the intrafusal muscle fibers contract, a precise amount of tension is reset within the muscle spindle to stay sensitive to any stretch that’s placed on the muscle. The gamma motor neurons play a crucial role in the muscle stretch reflex mechanism, working alongside the alpha motor neurons. This mechanism, which is sensitive to muscle stretch, enables precise adjustments to muscle length and velocity, contributing to the maintenance of muscle tone. Changes in the functioning of these pathways can impact either the threshold for activation or the size of the reflex (Michael-Titus et al., 2010). Several techniques can strengthen the amplitude of the reflex. These include clenching the teeth, performing the Jendrassik maneuver by interlocking fingers and pulling, and diverting attention from the reflex. 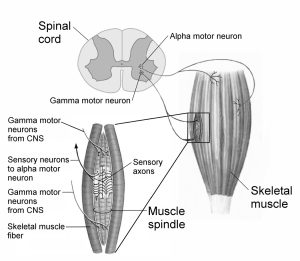
https://somaticmovementcenter.com/gamma-loop/?locale=en
Muscle Receptors & Proprioception
For proper functioning, the motor system relies on sensory input, including information about the external environment and the current state of muscles and limbs (learn more in chapter 2). The sense of proprioception helps in determining the body’s position in space by utilizing specialized receptors present in muscles and tendons. In-order to assist in proprioception, the muscle spindle provides information about changes in the length of a muscle, while the Golgi tendon organ signals the amount of force being exerted on a muscle. Both are essential for proper functioning of muscle contractions and relaxation.
Muscle Spindles
Muscle spindles are regarded as the most important proprioceptors (Kröger & Watkins, 2021). They are present in almost every muscle and are extremely sensitive receptors that relay information to the central nervous system. The information relayed includes the alterations in length of individual muscles and the speed in which it’s stretched. Despite their important role in proprioception, muscle spindles are relatively low in mass compared to the large muscle in which it resides.(Kröger & Watkins, 2021). It’s also estimated that there are 50,000 muscle spindles in the human body (Banks & Barker, 2004; Kröger & Watkins, 2021). Interestingly, muscle spindles are largely absent in human facial muscles and are frequently found in slow twitch (type 1) muscle fibers (Cobo et al., 2017; Kröger & Watkins, 2021; Webb, 2017)!
Muscle spindles are enclosed in afferent (sensory) receptors and consist of intrafusal fibers that innervate the muscles (Kröger & Watkins, 2021). These sensory receptors allow the muscle spindles to detect stretch through efferent (motor) innervation, whether it’s lengthened or shortened. As the muscle is stretched, the muscle spindles are relayed the information and the intrafusal fibers are also stretched in a similar way. In-order to properly respond to this change in length, both nuclear bag fibers and nuclear chain fibers (types of intrafusal fibers) will respond immediately to rapid stretching (Webb, 2017). The rate in which these fibers are fired is proportional to the change in muscle length; a greater stretch of the muscle equates to an increased rate of firing. Furthermore, once detecting stretch, the primary and secondary afferent receptors are stimulated, which leads to an initiation of action potentials (electrical signals) to the spinal cord and activating the alpha motor neurons. The alpha motor neurons in return activate the extrafusal fibers of the muscle which causes the muscle to contract!
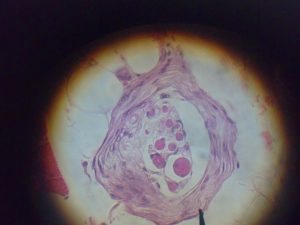
(Light microscope photograph of a muscle spindle)
Golgi Tendon Organ
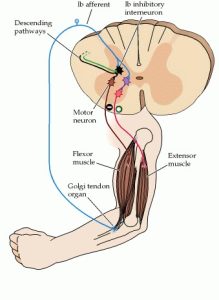
Along with the muscle spindles, skeletal muscles have a secondary type of sensory organ called the golgi tendon organ. On the other hand, unlike the muscle spindles which are found on the muscle itself, the golgi tendon organ is found in the tendons (Michael-Titus et al., 2010). In more detail, the golgi tendon organ is actually found in series with the muscle fiber and its primary function is to measure tendon tension. Tendon tension can be placed through either stretching or contraction or through the signal of muscle contraction (Webb, 2017). When tension is increased, an autogenic (negative) feedback reflex of the muscle called the inverse myotactic reflex is activated. This is a crucial reflex as it prevents the injury of the tendon through excessive tension. When a high level of tension is placed on the tendon, the golgi tendon organ stops activity in the muscles to prevent injury.
Golgi tendon organs are made of collagen fibres which contain Ib afferent neurons (sensory axons) at the intersection of the tendon and muscle (Michael-Titus et al., 2010). Golgi tendon organs are not as sensitive to passive stretch since most of the length changes from the muscle fibers, but sensitive to active contraction when the tendon experiences direct force because the collagen fibers experience tension (Michael-Titus et al., 2010; Purves et al., 2001). When the collagen fibers are stretched, the Ib afferent neurons fire and firing rate is proportional to the degree of tension (Michael-Titus et al., 2010). After the Ib afferent neurons are fired, it synapses with the interneurons or neurons that transmit signals between sensory and motor neurons. These interneurons then synapse with alpha motor neurons of inhibitory and facilitatory muscles to inhibit the movement and cause the muscle to relax!
Muscle Force
The amount of force exerted by muscle fibers is regulated by a motor neuron, and this relationship is guided by two fundamental principles: the rate code and the size principle.
Rate Code
During voluntary contraction, rate coding is one mechanism the central nervous system utilizes for greater muscular force (Deschenes, 1989). Motor neurons that are already stimulated are utilized by increasing the frequency of neural impulses. Furthermore, this increase in muscular force is caused without actually recruiting more motor units! Rate coding is also the primary source of fast voluntary muscle contraction such as a boxer throwing a quick jab. The already activated motor neurons communicate the desired level of force or intensity of a movement to the muscles (Enoka & Duchateau, 2017). In other words, when the motor neuron fires, it sends a signal (action potential) to the muscle to contract, and the frequency of these signals determines the level of force produced by the muscle. It’s important to note, that the central nervous system first recruits motor units when producing muscular force, but once a specific threshold is reached, rate coding allows for an even further increase in muscular force through increased frequency of neural impulses (Deschenes, 1989). Through rate coding, the motor system is able to produce additional muscular force, otherwise unachievable through solely the recruitment of motor units.
Henneman’s Size Principle
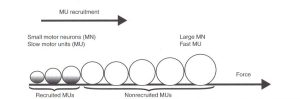
Henneman’s size principle is another concept in motor control that describes how motor units (a motor neuron and the muscle fibers it innervates) are recruited to produce force. Starting in 1957, a series of papers by Elwood Henneman suggested the term “size principle” through novel discoveries in the physiology community (Mendell, 2005). The principle states that motor units are recruited in order of their Henneman’s size principle, with smaller motor units being recruited first, followed by larger motor units. This means that when a low level of force is required, smaller motor units are activated, and as the required force increases, larger motor units are recruited. The size of a motor unit is determined by the number of muscle fibers it innervates. Smaller motor units innervate a small number of muscle fibers, while larger motor units innervate a larger number of muscle fibers. A common example of the size principle is seen in the recruitment of motor units during a bicep curl exercise.
During the beginning of the exercise, when the weight is light, the smaller motor units with low thresholds in the bicep muscle are recruited first (Deschenes, 1989). These motor units innervate smaller muscle fibers, and are able to produce force with less energy. As the weight of the curl increases, larger motor units are recruited to produce the necessary force to lift the weight. These larger motor units innervate larger muscle fibers and require more energy to produce force. However, as larger motor units with higher thresholds are recruited and maxed out, a further increase in muscular force is primarily achieved through rate coding (Deschenes, 1989).
The size principle ensures that the motor system is able to produce the appropriate amount of force for a given task with maximum efficiency. Small motor units are more easily recruited and are able to produce force with less energy, while larger motor units are recruited when higher forces are required. Without the size principle, the muscle would have to recruit all of its motor units simultaneously, which would require tremendous amounts of energy and could lead to fatigue quickly. Additionally, the size principle plays a role in the development of muscle strength. In the strength & conditioning and sports performance world, the size principle is frequently applied. Training with heavier weights, for example, can recruit larger motor units and increase the size of the corresponding muscle fibers, leading to greater muscle strength.
Make a fist and start to squeeze your fingers slowly without much force. Are small or large motor units primarily recruited? That’s right, the smaller motor units innervate smaller muscle fibers that produce less force.
Now, make a fist and squeeze your fingers quickly and tightly with as much force. You just recruited larger motor units! These motor units innervate larger muscle fibers that produce more force.
Muscle Fiber Types and Motor Neurons
Like previously mentioned, motor neurons innervate muscle fibers to form contractions. Interestingly, depending on the size of the motor neuron, the type of muscle it innervates differs as well (Kraemer & Looney, 2012). Small motor neurons innervate slow-twitch fibers, intermediate sized motor neurons innervate fast twitch, fatigue resistant fibers and large motor neurons innervate fast twitch, fatigable fibers (Knierim, 2020). Slow twitch muscle fibers are unable to generate large amounts of force, but can maintain force for an extended period (Mukund & Subramaniam, 2019). Slow twitch fibers have high levels of mitochondrial enzymes and exhibit low glycolytic activity. These types of fibers are utilized for maintaining posture. On the other hand, fast twitch muscle fibers contract at a faster rate, support anaerobic activity, but cannot be maintained for prolonged periods. These fiber types can be seen used during high intensity activities such as a short burst of sprint.
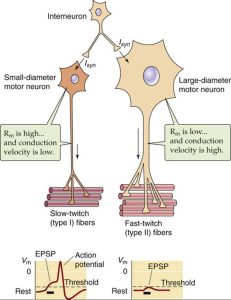
(Kandel et al., 2000)
Putting It All Together
Motor output is a fascinating process in the human body that allows individuals to move both voluntarily and involuntarily through a wide array of sensory input. Beginning with the types of motor neurons, alpha motor neurons and gamma motor neurons initiate voluntary or involuntary contractions through the innervations of different types of muscles (intrafusal & extrafusal). Additionally, if activated together through alpha gamma coactivation, a reflex can occur by working with the muscle spindles. Interestingly, the muscle spindle and golgi tendon sensory organs exist on the muscle itself and provide the sensory information needed for movement and reactions! The degree and force to which these movements and reactions occur are regulated through rate coding and the Henneman’s Principle, which serve as a mechanism to initiate different muscle fibers!
Please provide your feedback here
References
Includes the brain and spinal cord, which work together to process and integrate sensory information and generate appropriate motor commands.
Type of nerve cell that is responsible for controlling voluntary and involuntary muscle movement
Instruct the muscles to contract and generate movement in response to various stimuli, such as touch, sound, or visual cues.
Innervate and stimulate skeletal muscle to produce contractions, which result in movement
Type of motor neuron that are involved in regulating the contraction of intrafusal muscle fibers, which are found within a specialized sensory organ called the muscle spindle
Occurs when the alpha and gamma motor neurons are stimulated simultaneously
The sense or perception of the position, movement, and spatial orientation of one's body and its limbs
Specialized sensory receptor found within skeletal muscles that is responsible for monitoring changes in muscle length and providing proprioceptive feedback to the central nervous system
Sensory receptor found in tendons, which are the tough bands of connective tissue that attach muscles to bones.
Neurological reflex that helps to regulate muscle tension and prevent excessive force generation
Type of protein fiber found in connective tissue, including tendons, ligaments, and cartilage
Type of neuron that function as intermediaries between sensory neurons, motor neurons, and other interneurons
Mechanism used by neurons to transmit information about the intensity of a stimulus by modulating the frequency of action potentials, or spikes, that they generate.
Order in which motor units are recruited during muscle contractions
Type of skeletal muscle fiber that contract slowly
Type of skeletal muscle fiber that contract rapidly and generate high levels of force